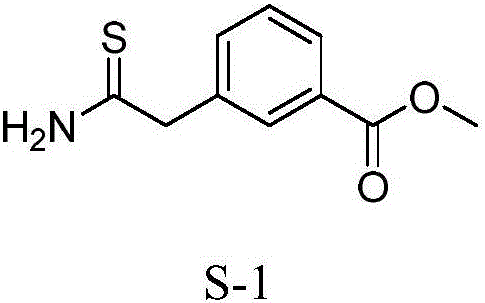
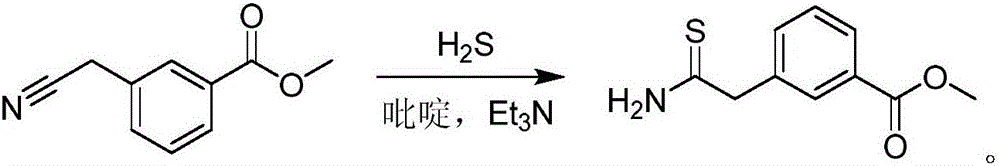
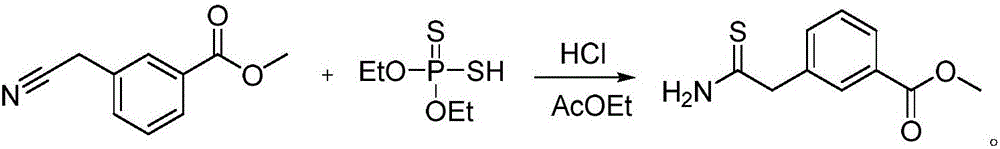Synthesis method of 3-(2-amino-2-thioethyl)methyl benzoate
A technology of methyl cyanomethyl benzoate and methyl benzoate, which is applied in the field of synthesizing methyl 3-benzoate, can solve problems such as strong organic weak acid odor, influence on catalytic effect, and easy volatilization of secondary amines, and achieve high product quality , fast reaction rate, simple post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1, a kind of synthetic method of 3-(2-amino-2-thioethyl) methyl benzoate, carries out following steps successively:
[0050] 1), add 3-cyanomethylbenzoic acid methyl ester 35.3g (99.2wt%, 0.2mol), 70wt% (mass %) NaSH (24.0 g, 0.3mol) and methanol 80ml, the water bath is heated to about 70 ± 5 ° C, under the condition of mechanical stirring, the methanol solution (200ml) of triethylamine hydrochloride (41.3g, 0.3mol) is slowly added dropwise, and the addition time is 6h, continue to stir for 1h after the addition, and stop the reaction (the reaction process is tracked and detected by HPLC, when the remaining raw materials are less than 1%, stop the reaction).
[0051]2), after the reaction finishes, filter; Filter residue is NaCl, thereby realizes removing the NaCl that reaction generates; The filtrate is distilled (distillation temperature is respectively 68 ℃ and 90 ℃) thereby reclaims methanol (250ml) and triethylamine (38ml, about 0.27mol), the residue af...
Embodiment 2
[0052] Embodiment 2, a kind of synthetic method of 3-(2-amino-2-thioethyl) methyl benzoate, carry out following steps successively:
[0053] 1), add 3-cyanomethylbenzoic acid methyl ester 35.3g (99.2wt%, 0.2mol), 70wt% NaSH (24.0g, 0.28mol) in the 500ml four-neck flask that thermometer, reflux condenser and stirrer are equipped ) and methanol 80ml, the water bath temperature was raised to about 70±5°C, under the condition of mechanical stirring, slowly dropwise the methanol solution (200ml) of di-n-propylamine hydrochloride (38.5g, 0.28mol), the feeding time was 6h, and the feeding was finished After that, the stirring was continued for 1 h, and the reaction was stopped (the reaction process was tracked and detected by HPLC, and when the remaining raw material was less than 1%, the reaction was stopped).
[0054] 2), after the reaction is finished, filter; remove the NaCl generated by the reaction, distill the filtrate so as to reclaim methanol and di-n-propylamine, the still ...
Embodiment 3
[0055] Embodiment 3, a kind of synthetic method of 3-(2-amino-2-thioethyl) methyl benzoate, carries out following steps successively:
[0056] 1), add 3-cyanomethylbenzoic acid methyl ester 35.3g (99.2wt%, 0.2mol), 70wt% NaSH (24.0g, 0.3mol) in the 500ml four-necked flask that thermometer, reflux condenser and stirrer are equipped with ) and methanol 80ml, the temperature of the water bath was raised to about 70±5°C, under the condition of mechanical stirring, the methanol solution (200ml) of diisopropylamine hydrochloride (41.3g, 0.3mol) was slowly added dropwise, the feeding time was 6h, and the feeding was completed After that, the stirring was continued for 1 h, and the reaction was stopped (the reaction process was tracked and detected by HPLC, and when the remaining raw material was less than 1%, the reaction was stopped).
[0057] 2), after the reaction is finished, filter; remove the NaCl generated by the reaction, distill the filtrate to recover methanol and diisoprop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com