Controllable preparation of cu2znsns4 nanocrystalline materials
A technology of nanocrystalline material and copper acetate, which is applied in the field of nanomaterials and nanometers, can solve the problems of long reaction time and high temperature, and achieve the effect of simple process, low cost and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Cu 2 ZnSnS 4 The controllable preparation method of nanocrystalline material comprises the following steps:
[0027] (1) Add 2mmol copper acetate, 1mmol zinc acetate, and 1mmol tin tetrachloride to 20mL oleylamine, pass in argon protection gas, control the temperature at 50°C, and stir rapidly for 5 minutes to make copper acetate, zinc acetate 1. The tin tetrachloride is completely dissolved to obtain a complex solution of the metal salt;
[0028] (2) Add 3 mL of a diphenyl ether solution with a concentration of 1 mol / L diphenylthiourea to the above metal salt solution, heat to a set temperature of 100°C and react for 1 minute;
[0029] (3) After the reaction is over, cool to room temperature, add a large amount of methanol to the Cu obtained by the reaction 2 ZnSnS 4 Nanocrystals are cleaned to obtain Cu 2 ZnSnS 4 nanocrystalline material.
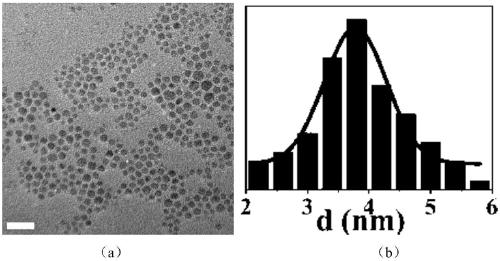
[0030] This embodiment prepares Cu 2 ZnSnS 4 The particle size of the nanocrystalline material is 1.9nm, and its TEM ima...
Embodiment 2
[0032] Cu 2 ZnSnS 4 The controllable preparation method of nanocrystalline material comprises the following steps:
[0033] (1) Add 2mmol copper acetate, 1mmol zinc acetate, and 1mmol tin tetrachloride to 20mL oleylamine, pass in argon protection gas, control the temperature at 60°C, and stir rapidly for 10 minutes to make copper acetate, zinc acetate 1. The tin tetrachloride is completely dissolved to obtain a complex solution of the metal salt;
[0034] (2) Add 4 mL of a diphenyl ether solution with a concentration of 1 mol / L diphenylthiourea to the above metal salt solution, heat to a set temperature of 140°C and react for 5 minutes;
[0035] (3) After the reaction is over, cool to room temperature, add a large amount of methanol to the Cu obtained by the reaction 2 ZnSnS 4 Nanocrystals are cleaned to obtain Cu 2 ZnSnS 4 nanocrystalline material.
[0036] This embodiment prepares Cu 2 ZnSnS 4 The particle size of the nanocrystalline material is 3.9nm, and its TEM i...
Embodiment 3
[0038] Cu 2 ZnSnS 4 The controllable preparation method of nanocrystalline material comprises the following steps:
[0039] (1) Add 2mmol copper acetate, 1mmol zinc acetate, and 1mmol tin tetrachloride to 20mL oleylamine, pass in argon protection gas, control the temperature at 70°C, and stir rapidly for 15 minutes to make copper acetate, zinc acetate 1. The tin tetrachloride is completely dissolved to obtain a complex solution of the metal salt;
[0040] (2) Add 5 mL of a diphenyl ether solution with a concentration of 1 mol / L diphenylthiourea to the above metal salt solution, heat to a set temperature of 180°C and react for 10 minutes;
[0041] (3) After the reaction is over, cool to room temperature, add a large amount of methanol to the Cu obtained by the reaction 2 ZnSnS 4 Nanocrystals are cleaned to obtain Cu 2 ZnSnS 4 nanocrystalline material.
[0042] This embodiment prepares Cu 2 ZnSnS 4 The particle size of the nanocrystalline material is 5.5nm, and its TEM im...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com