Tuberculosis immunodiagnosis molecular marker and application thereof in preparation of vaccines
A technology for tuberculosis and Mycobacterium tuberculosis, applied in the fields of immunology and molecular biology, can solve the problems of easy false positive rate, can not be used as a diagnostic basis, and easy to be misdiagnosed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: Mass spectrometry identification experiment
[0047] 1. Experimental Materials and Instruments
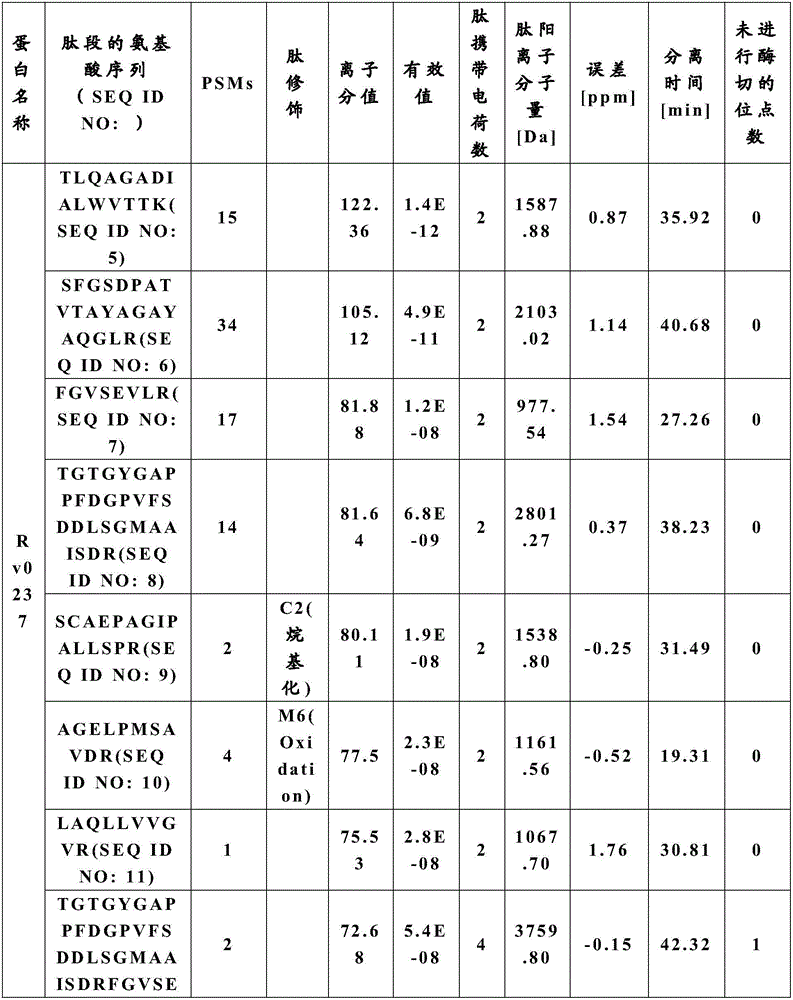
[0048]Target protein samples: prepared Rv0237 protein (SEQ ID NO:1) and Rv1111c protein (SEQ ID NO:2).
[0049] Instruments: Ultimate 3000Nano-LC system (Dionex, USA), linear ion trap multistage tandem mass spectrometer (linear trap quadruple, LTQ), Orbitrap Velos mass spectrometer (ThermoScientific, Germany).
[0050] Data processing software: MassLynx version 4.1 (Dionex, USA), Xcalibur software v2.2.6 (Thermo), MASCOT version 2.2 (Matrix Sciences, UK).
[0051] 2. Experimental method
[0052] (1) After the target protein is subjected to SDS PAGE, rinse the film with deionized water for 15 minutes; cut off the target fragment, 1-2 mm in size; put it in a centrifuge tube with low protein adsorption, and use 100 μL of ddH 2 O Rinse the pellets in the dish and repeat, discard the liquid and add 40 μL of (ACN) / ddH 2 O(50 / 50), discard the liquid, incubate for...
Embodiment 2
[0075] Embodiment 2: Humoral immunity experiment
[0076] Humoral immune antigen can specifically bind with the body produced, prevent it from infecting normal cells, and combine with macrophages, so that macrophages can phagocytize antigens to achieve the purpose of sterilization, so proteins with high humoral immune response intensity can also be Stimulate the body to produce antibodies to achieve immune protection.
[0077] 1. Experimental materials
[0078] Protein samples: Rv0237 protein and Rv1111c protein. Positive control 38kDa (Immuno Diagnostics Inc. USA).
[0079] Main reagents: mouse secondary antibody high-throughput kit, PS-MK15 Wes-Mouse 12-230 kDa master kit with split buffer for 200 data (Immuno Diagnostics Inc. USA). Streptomycin-labeled goat anti-human IgG, Thermo Scientific, Germany).
[0080] 2. Experimental principles and methods
[0081] Using Western Blot method. Western Blot technology is a classic protein detection technology. The traditional ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com