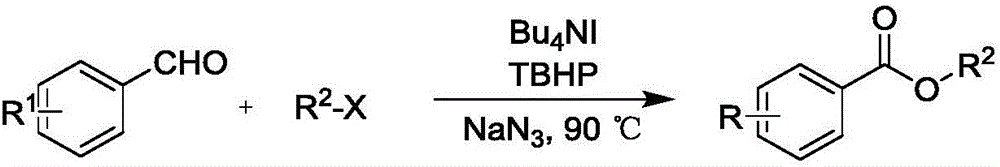
Synthesis method of carboxylic ester compound
A technology of ester compounds and synthetic methods, applied in the preparation of organic compounds, carboxylic acid ester preparation, chemical instruments and methods, etc., can solve the problems of strong acid and strong alkali, unfavorable environmental protection, toxic and harmful heavy metal residues, difficult separation of products and reagents, etc. problems, to achieve good response characteristics, low equipment requirements, and good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: the preparation of benzyl 4-methoxybenzoate
[0051] Take a clean 15mL high-pressure reaction bottle, add p-methoxybenzaldehyde (74.8mg, 0.55mmol), tetrabutylammonium iodide (40.6mg, 0.11mmol), sodium azide (107.25mg, 1.65mmol), oxidant tert Butyl hydroperoxide (0.10mL, 0.825mmol) and benzyl chloride (139.24mg, 1.1mmol), solvent toluene (1.0mL), add small magnets, react at 100°C for 2h, extract with dichloromethane (3×10mL) , the organic phase was desolvated under reduced pressure, using ethyl acetate:petroleum ether=1:30 solvent as eluent, 300 mesh column chromatography silica gel as filler, and the target product (118.50mg, yield 89% obtained by column chromatography separation and purification) %).
[0052] 1 HNMR (500MHz, CDCl 3 ):δ=8.04-8.02(m,2H),7.44-7.43(m,2H),7.39-7.36(m,2H),7.34-7.31(ddd,1H),5.33(s,2H),3.83(s ,3H); 13 CNMR (125MHz, CDCl 3 ): δ=166.21, 163.47, 136.34, 131.77, 128.59, 128.16, 128.12, 122.57, 113.65, 66.41, 55.43ppm;
Embodiment 2
[0053] Embodiment 2: the preparation of methyl 4-methoxybenzoate
[0054] Take a clean 15mL high-pressure reaction bottle, add p-methoxybenzaldehyde (74.8mg, 0.55mmol), tetrabutylammonium iodide (40.6mg, 0.11mmol), sodium azide (107.25mg, 1.65mmol), oxidant tert Butyl hydroperoxide (0.10mL, 0.825mmol), and iodomethane (156.15mg, 1.1mmol) solvent toluene (1.0mL), add a small magnet, react at 90°C for 2h, and extract with dichloromethane (3×10mL) , the organic phase was desolvated under reduced pressure, using ethyl acetate:petroleum ether=1:30 solvent as the eluent, 300 mesh column chromatography silica gel as the filler, and the target product obtained by column chromatography separation and purification (76.71mg, yield 84 %).
[0055] 1 H NMR (500MHz, CDCl 3 ):δ=8.00-7.98(d,2H),6.92-6.91(d,2H),3.88(s,3H),3.86(s,3H); 13 C NMR (125MHz, CDCl 3 ): δ=166.88, 163.33, 131.51, 122.61, 113.60, 55.41, 51.86ppm.
Embodiment 3
[0056] Embodiment 3: the preparation of ethyl 4-methoxybenzoate
[0057] Take a clean 15mL high-pressure reaction bottle, add p-methoxybenzaldehyde (74.8mg, 0.55mmol), tetrabutylammonium iodide (40.6mg, 0.11mmol), sodium azide (107.25mg, 1.65mmol), oxidant tert Butyl hydroperoxide (0.10mL, 0.825mmol), and ethyl bromide (119.86mg, 1.1mmol), solvent toluene (1.0mL), add small magnets, react at 90°C for 2h, dichloromethane (3×10mL ) extraction, the organic phase decompression precipitation, with ethyl acetate:petroleum ether=1:30 solvent is eluent, 300 mesh column chromatography silica gel is filler, the target product (83.19mg that obtains by column chromatography separation and purification, obtains rate of 84%).
[0058] 1 H NMR (500MHz, CDCl 3 ):δ=8.01-7.99(d,2H),6.92-6.90(d,2H),4.37-4.32(q,2H),3.85(s,3H),1.39-1.36(t,1H); 13 C NMR (125MHz, CDCl 3 ): δ=166.41, 163.25, 131.53, 125.92, 122.96, 114.01, 113.54, 60.63, 55.40, 14.38ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com