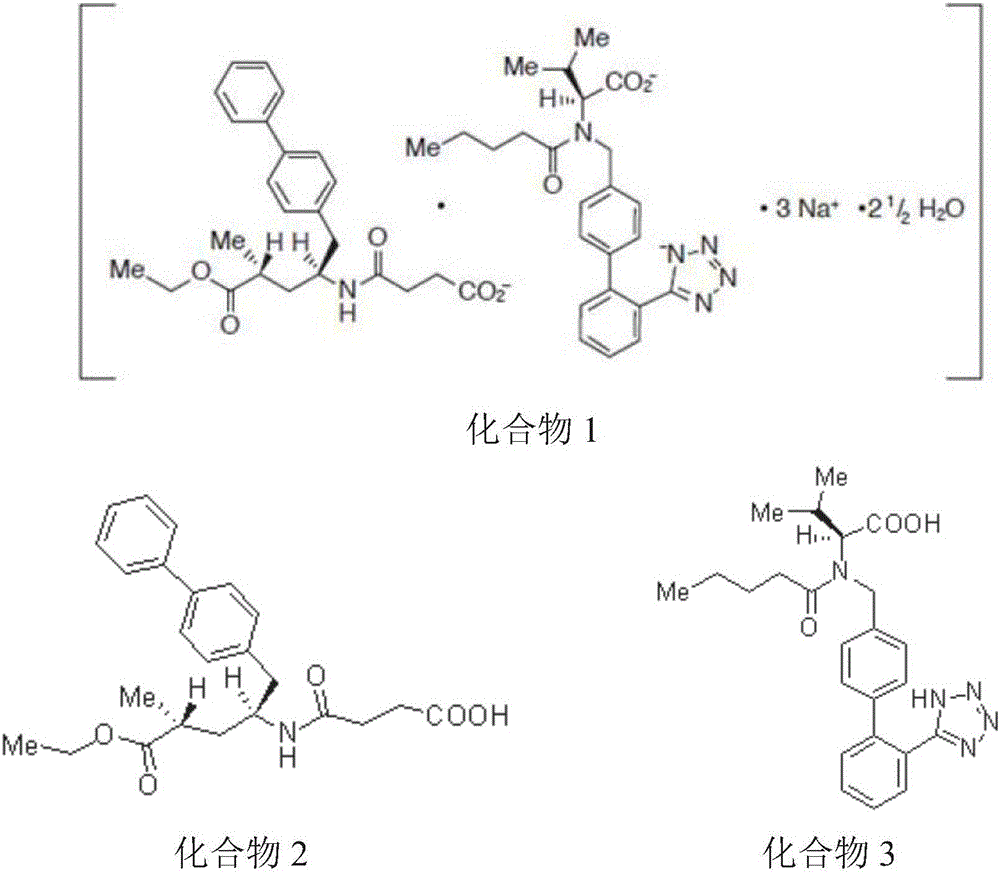
Oral preparation for cardiovascular disease treatment, and preparation method thereof
A technology for oral preparations and disintegrants, applied in the field of pharmaceutical preparations, can solve problems such as undisclosed technical parameters, unsuggested technological level, etc., and achieves the effects of good dissolution performance, good fluidity, and improved preparation quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
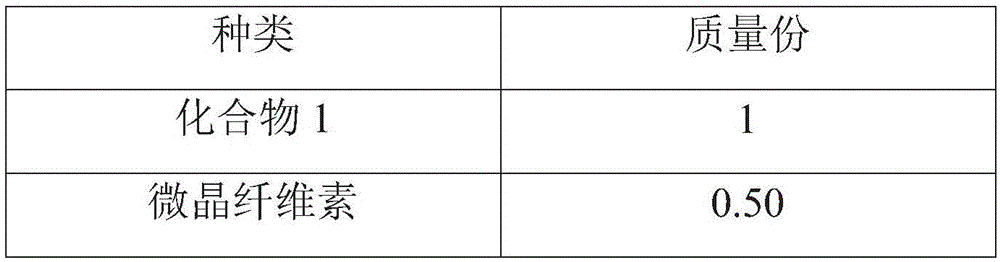
[0064] prescription:
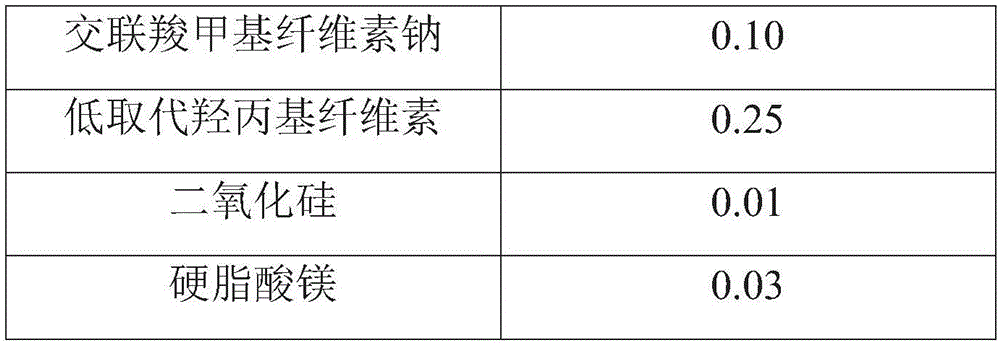
[0065]
[0066] Preparation:
[0067] 1. Pass the original and auxiliary materials through a 40-mesh sieve, and set aside;
[0068] 2. Take the raw and auxiliary materials and mix them to obtain the inner granule total blend powder;
[0069] 3. Use a dry granulator to compress the premixed mixture with a pitch of 0.7mm, a speed of 4r / min, and a feeding speed of 24r / min to obtain a density of 0.97g / cm 3 blockbuster;
[0070] 4, the blockbuster through Obtain internal phase granule (angle of repose 32.1 °) after sieving granulation;
[0071] 5. Mix the internal phase granules and the external auxiliary materials for 10 minutes, mix evenly (RSD value is 1.2%), and compress the obtained mixture to obtain a tablet core of compound 1 (tablet hardness 6.0-10.0kgf);
[0072] 6. The obtained tablet core is coated with the Opadry coating polymer to obtain a coated tablet.
[0073] After testing, the difference in weight of the obtained tablets was ±2.2%....
Embodiment 2
[0075] prescription:
[0076]
[0077]
[0078] Preparation:
[0079] 1. Pass the original and auxiliary materials through a 40-mesh sieve, and set aside;
[0080] 2. Take the raw and auxiliary materials and mix them evenly to obtain the mixed powder of inner particles;
[0081] 3. Use a dry granulator to compress the premixed mixture with a pitch of 0.8mm rollers, a rotating speed of 3r / min rollers, and a feeding speed of 25r / min to obtain a density of 1.19g / cm 3 blockbuster;
[0082] 4, the blockbuster through Obtain internal phase granule (angle of repose 33.3 °) after sieving granulation;
[0083] 5. Mix the internal phase granules and the external auxiliary materials for 10 minutes, mix evenly (RSD value is 1.8%), and compress the obtained mixture to obtain a tablet core of compound 1 (tablet hardness 6.0-10.0kgf);
[0084] 6. The obtained tablet core is coated with the Opadry coating polymer to obtain a coated tablet.
[0085] After testing, the difference i...
Embodiment 3
[0087] prescription:
[0088]
[0089]
[0090] Preparation:
[0091] 1. Pass the original and auxiliary materials through a 40-mesh sieve, and set aside;
[0092]2. Take the raw and auxiliary materials and mix them to obtain the inner granule total blend powder;
[0093] 3. Use a dry granulator to compress the premixed mixture with a pitch of 0.5 mm rollers, a speed of 3 r / min, and a feeding speed of 20 r / min to obtain a density of 1.05 g / cm 3 blockbuster;
[0094] 4, the blockbuster through Obtain internal phase granule (angle of repose 30.8 °) after sieving granulation;
[0095] 5. Mix the internal phase granules and the external auxiliary materials for 10 minutes, mix evenly (RSD value is 1.3%), and compress the obtained mixture to obtain a tablet core of compound 1 (tablet hardness 6.0-10.0kgf);
[0096] 6. The obtained tablet core is coated with the Opadry coating polymer to obtain a coated tablet.
[0097] After testing, the difference in weight of the obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com