Triamcinolone tablet and polymorphs and preparation method
A technology of Xilong tablet and Longjing, which is applied in the field of steroids, can solve the problems of increased energy consumption, breakage, poor quality, and the need to stir for a long time to achieve uniform content when mixing with excipients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0029] Specific implementation method: X-ray powder diffraction test carried out in the embodiment of the present invention adopts X-ray wavelength λ=1.540598 ?
Embodiment 1
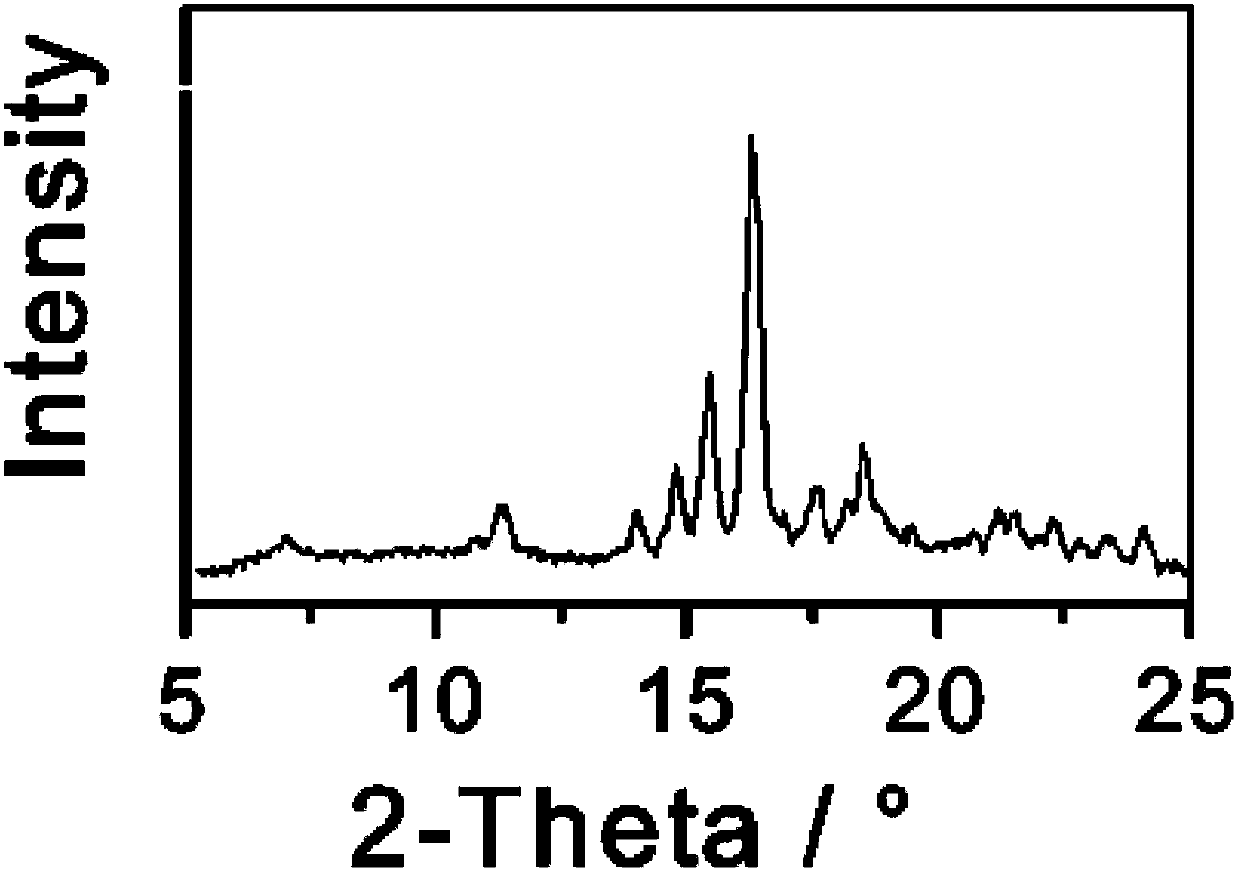
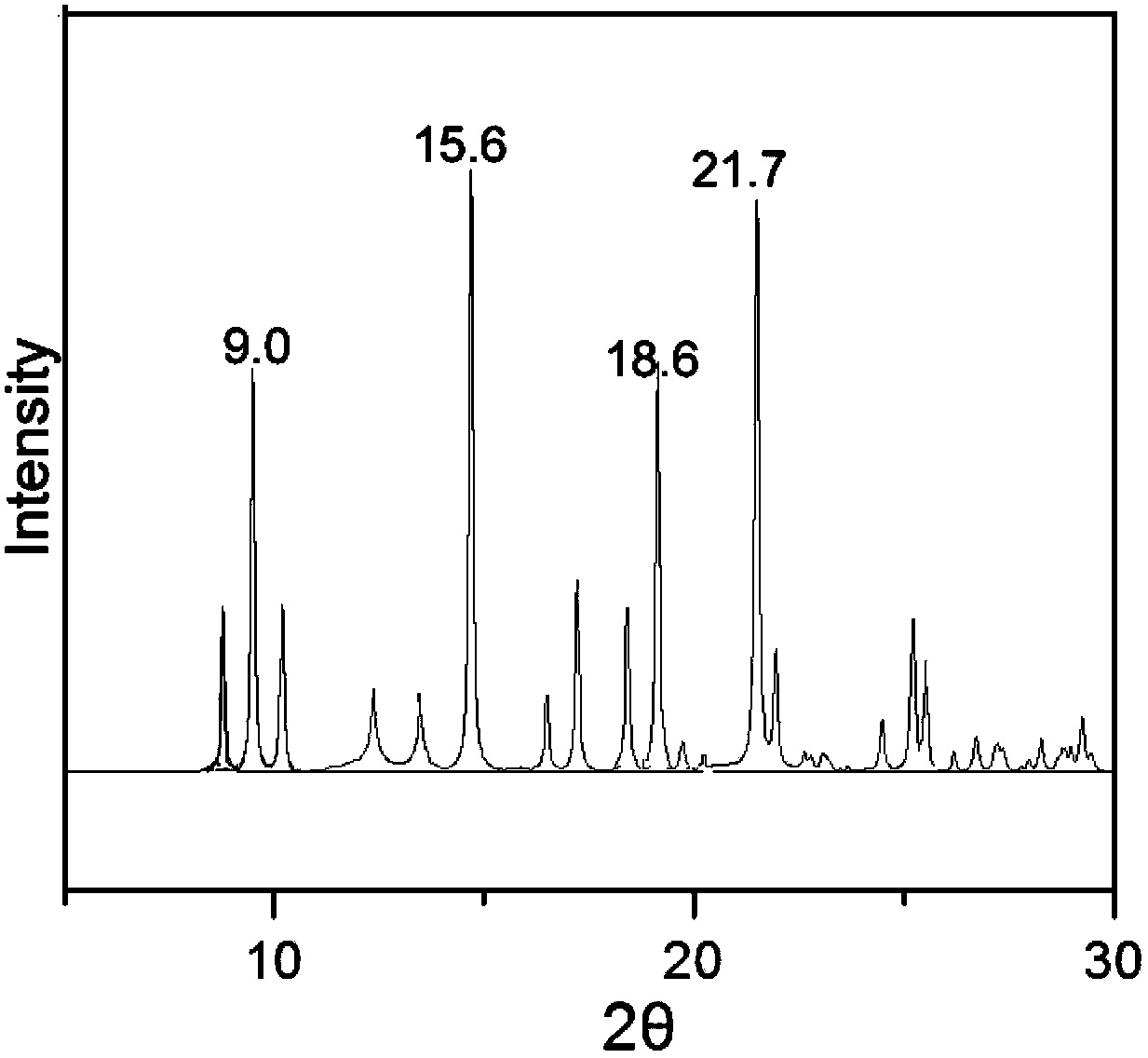
[0033] Dissolve 1 g of triamcinolone in 7 mL of butanol: tetrahydrofuran = 1:3 (volume ratio) mixed solvent, slowly add 50 ml of cyclohexane dropwise over 30 minutes, and slowly cool down to 0°C while adding cyclohexane dropwise. Keep stirring at 0°C for 1 h, filter and dry the precipitated crystals, and perform X-ray powder diffraction measurement. X-ray powder diffraction measurement, the measured characteristic peak positions are 2θ=9.0°, 15.6°, 18.6°, 21.7° The spectrogram is as follows image 3 shown.
Embodiment 2
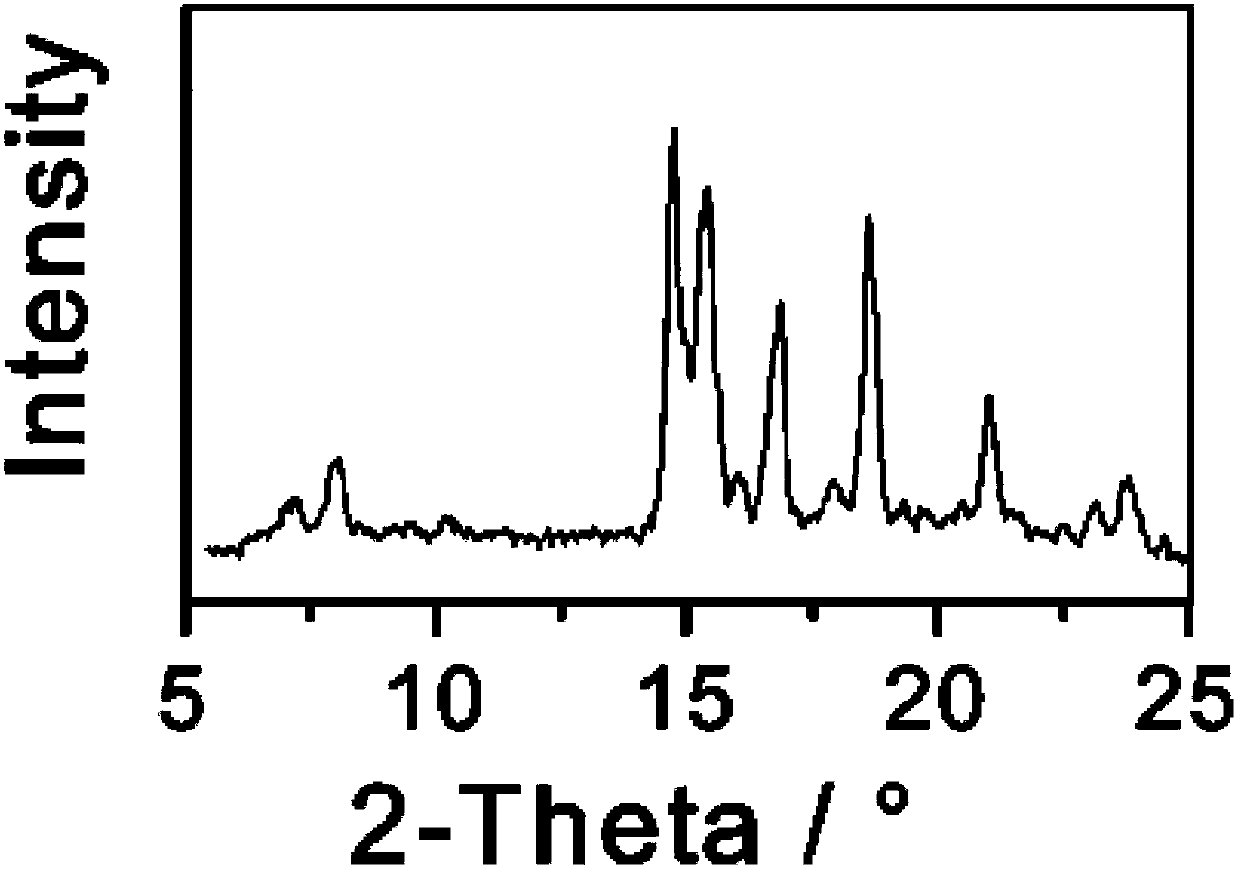
[0035] Take 1g of triamcinolone and dissolve it in 7mL of ethanol: tetrahydrofuran = 1:1 (volume ratio) mixed solvent, slowly add 50ml of n-hexane dropwise, and slowly cool down to 0°C while adding cyclohexane dropwise. Keep stirring at 0°C for 2 hours, filter and dry the precipitated crystals, and perform X-ray powder diffraction measurement. X-ray powder diffraction measurement, the measured characteristic peak positions are 2θ=8.9°, 15.6°, 18.6°, 21.8° The spectrogram is as follows Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com