Platinum catalyzed hydrosilylation reactions utilizing cyclodiene additives
A technology of hydrosilylation and cyclodiene, which is applied in the fields of compounds, organic chemistry, chemical instruments and methods of elements of group 4/14 of the periodic table, and can solve the problem of no COD-Pt substances, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1: Allyl Methacrylate (AMA) and SiMeCl 2 H Hydrosilylation using diene additives
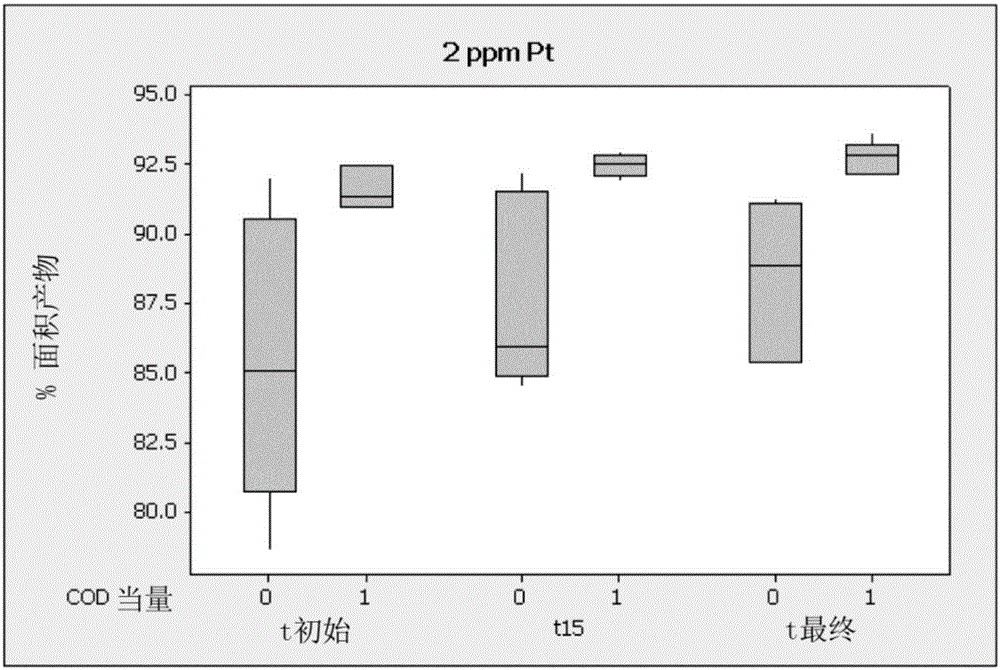
[0086] A 4-neck round bottom flask was fitted with an addition funnel, syringe port, alcohol thermometer, magnetic stir bar, and straight water condenser topped with a dry ice condenser. Addition funnel with N 2 inlet and before reacting the N 2 The tubing is separate from the t-piece that connects to the bubbler. The system uses N 2 Rinse and load with appropriate inhibitor. SiMeCl 2 H (16.5 g, 0.140 mol) was charged to the addition funnel. A solution of allyl methacrylate (19.7 g, 0.16 mol), chloroplatinic acid (2 ppm Pt) and cyclooctadiene (COD) in ethanol (0.39 μ mol, equimolar with Pt) was charged in the round bottom flask, Among them, COD is used as an additive. The reaction mixture was heated to 80 °C. Approximately 1 mL of chlorosilane was added and the reaction was monitored for exotherm. Once an exothermic reaction was detected, the chlorosilane was added slowl...
Embodiment 2
[0089] Example 2: Allyl Methacrylate with SiMeCl 2 H Hydrosilylation using a diene additive to evaluate the color of the reaction product
[0090] Reactions were performed similar to those discussed above with a 5 ppm Pt loading, except that no color-imparting inhibitor was added to the reaction. For allyl methacrylate and SiMeCl performed with different amounts of COD equivalents 2 The reaction of H evaluates the color of the product.
[0091] Table 1.
[0092] COD equivalent Color (Pt / Co) 0 331 0 193 0.5 150 1 46
Embodiment 3
[0093] Embodiment 3: Allyl methacrylate and Si(OEt) 3 H hydrosilylation with diene additive
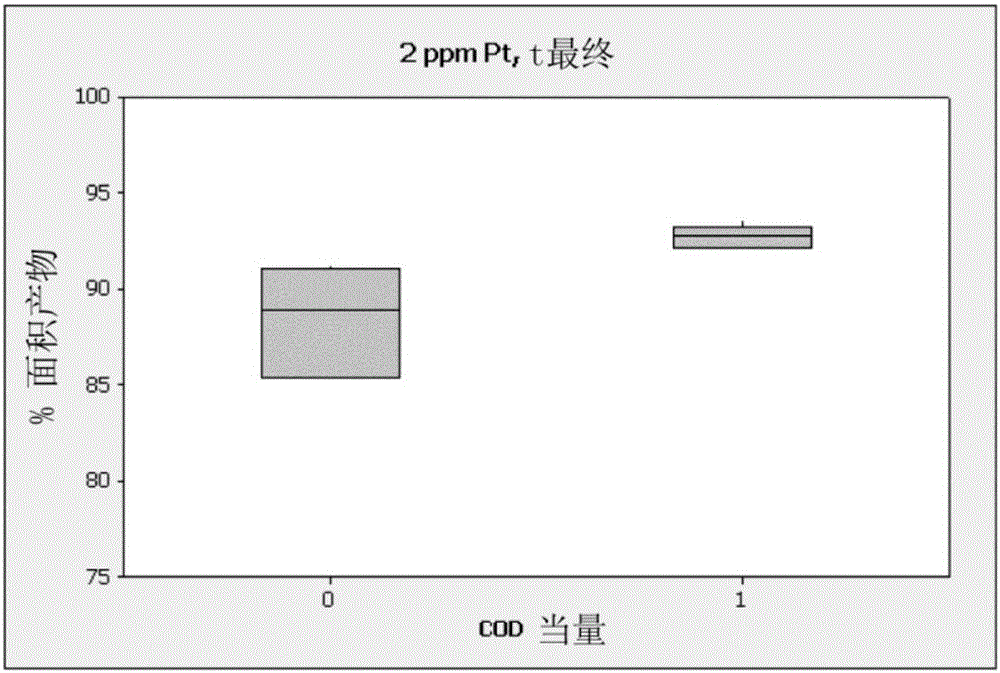
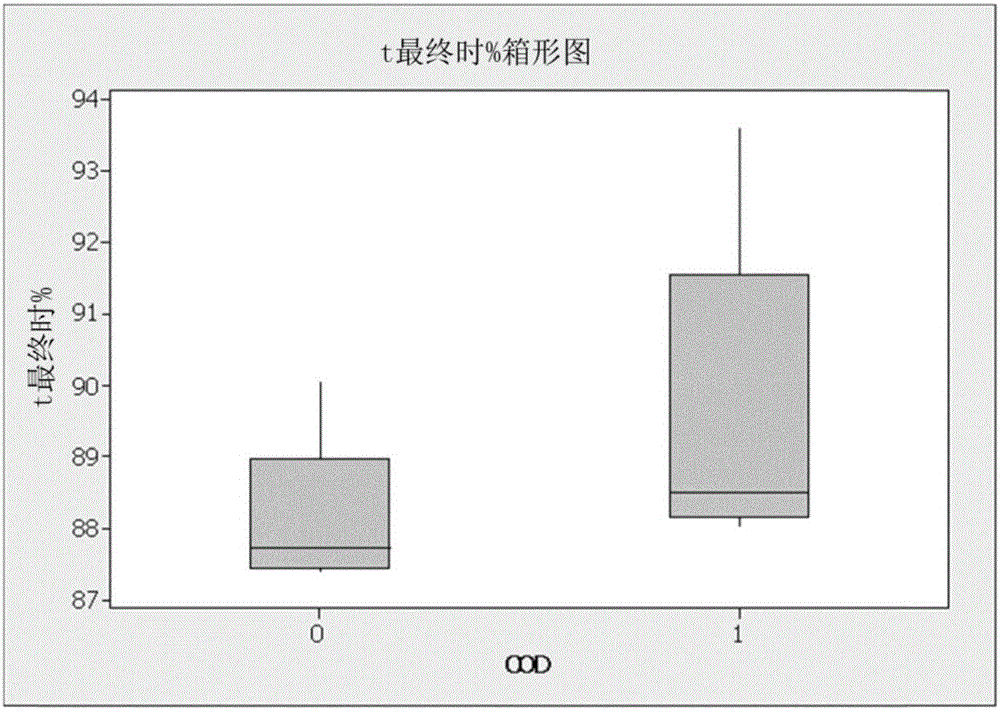
[0094] A 4-neck round bottom flask was equipped with a magnetic stir bar, a direct condenser with a dry ice condenser on top, a syringe port, a liquid addition funnel, and a thermometer and was placed under N 2 Down. A reaction vessel was charged with inhibitor and allyl methacrylate (15 mL, 0.11 mol), chloroplatinic acid solution (2 ppm Pt) COD solution (equimolar to Pt). The addition funnel was charged with triethoxysilane (19 mL, 0.1 mol). The reaction was heated to 90°C, then an aliquot of triethoxysilane (3-5 vol%) was added and the reaction was monitored for exotherm. After confirmation of the exotherm, the remaining triethoxysilane was added via the addition funnel at a rate to maintain the reaction at 85°C-95°C. After the final addition of triethoxysilane, the reaction was heated at 90°C for 90 minutes. by GC and 1 The resulting product was analyzed by H NMR. The averag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com