Synthetic method for crystal violet lactone
A technology of crystal violet lactone and its synthesis method, which is applied in the field of crystal violet lactone synthesis, and can solve the problems of unavoidable triphenylmethane, product yield impact, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
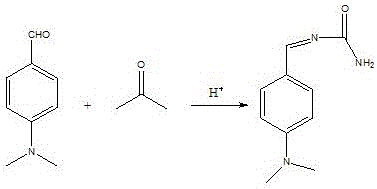
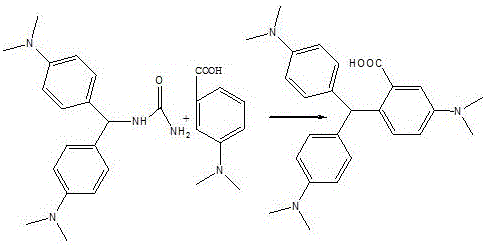
[0035] Dissolve 2.4g of urea in 10mL of water, slowly add 3mL of concentrated sulfuric acid dropwise to it, then add 3.07g of p-dimethylaminobenzaldehyde, raise the temperature to 50°C, react for 2h, then add 2.5mL of p-dimethylaniline to it, Raise the temperature to 80°C, keep it warm for 2 hours, add 3.33g p-dimethylaminobenzoic acid, raise the temperature to 90°C, keep it warm for 2h, then add 20mL of water, keep it warm for 8h, cool down to room temperature naturally, at 50°C, dropwise add 10g hydrogen Add 0.1g of copper sulfate pentahydrate to a solution composed of potassium oxide, 100mL of water and 6mL of ammonia water, heat up to 70°C, add dropwise a solution of 12mL of 30% hydrogen peroxide and 24mL of water, add dropwise for 2 hours, and cool down , filtered, and dried to obtain 6.8 g of the product.
Embodiment 2
[0037] (replace concentrated sulfuric acid with concentrated hydrochloric acid)
[0038] The experiment was carried out with concentrated hydrochloric acid instead of concentrated sulfuric acid as raw material, and other conditions were the same as in Example 1. 0.5 g of the product was obtained, and in the step of adding dropwise to the potassium hydroxide solution, a large amount of solid insoluble impurities appeared.
Embodiment 3
[0040] (The reaction temperature of the colorless crystal violet lactone stage is heating to reflux)
[0041] The synthesis experiment was carried out with the reaction temperature of the colorless crystal violet lactone stage being heated to reflux, and other conditions were the same as in Example 1. During the step of adding dropwise to the potassium hydroxide solution, a large amount of colloidal impurities appeared. The product 4.8g was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com