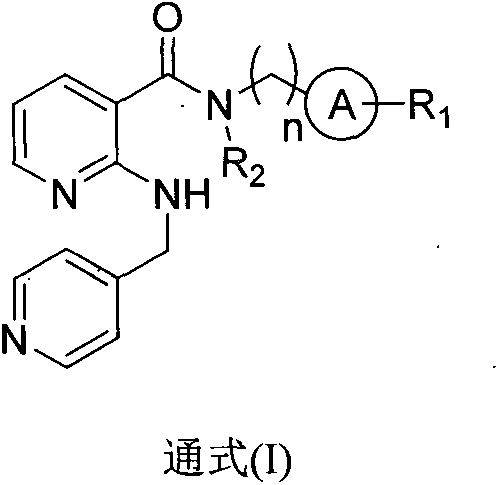
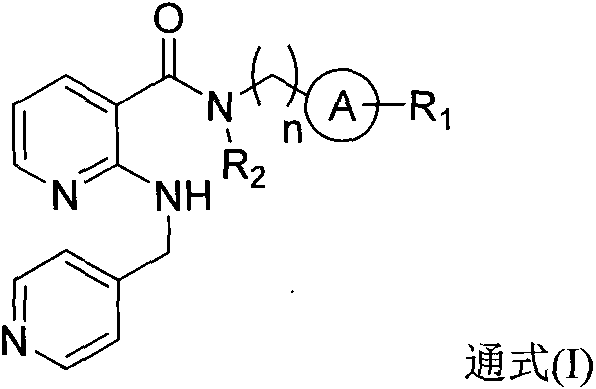
2-aminomethylpyridylnicotinamides and preparation method and application thereof
A compound, nicotinamide technology, applied in the field of medicinal chemistry, can solve the problem of no MDR reversal agent on the market
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Preparation of 2-((pyridine-4-methylene)amino)nicotinic acid (3)
[0085]
[0086] Add 2-chloronicotinic acid (25.3mmol), copper oxide (catalytic amount), and potassium carbonate (25.3mmol) into a 100ml round bottom flask, stir at room temperature for 20min, add 4-methylaminopyridine (50.6mmol), and heat at 110°C 2h. Then add ethyl acetate and stir to room temperature, filter with suction, wash the filter cake twice with ethyl acetate, dissolve in water (20ml), adjust to pH 5-6 with 4N hydrochloric acid, let stand to precipitate, filter with suction, dry the filter cake, The obtained crude product was further purified by hot beating with ethanol, and filtered with suction to obtain 5.16 g of off-white solid with a yield of 89% and a melting point of 197-199°C.
[0087] 1 H NMR (300MHz, DMSO-d 6 ) δppm: 8.61 (d, J=5.8Hz, 1.0H, ArH), 8.48 (s, 2H, ArH), 8.19 (dd, J=4.7, 1.9Hz, 1H, ArH), 8.11 (dd, J=7.7 , 1.9Hz, 1H, ArH), 7.29(d, J=4.7Hz, 2H, ArH), 6.64(dd, J=7.7, 4....
Embodiment 2
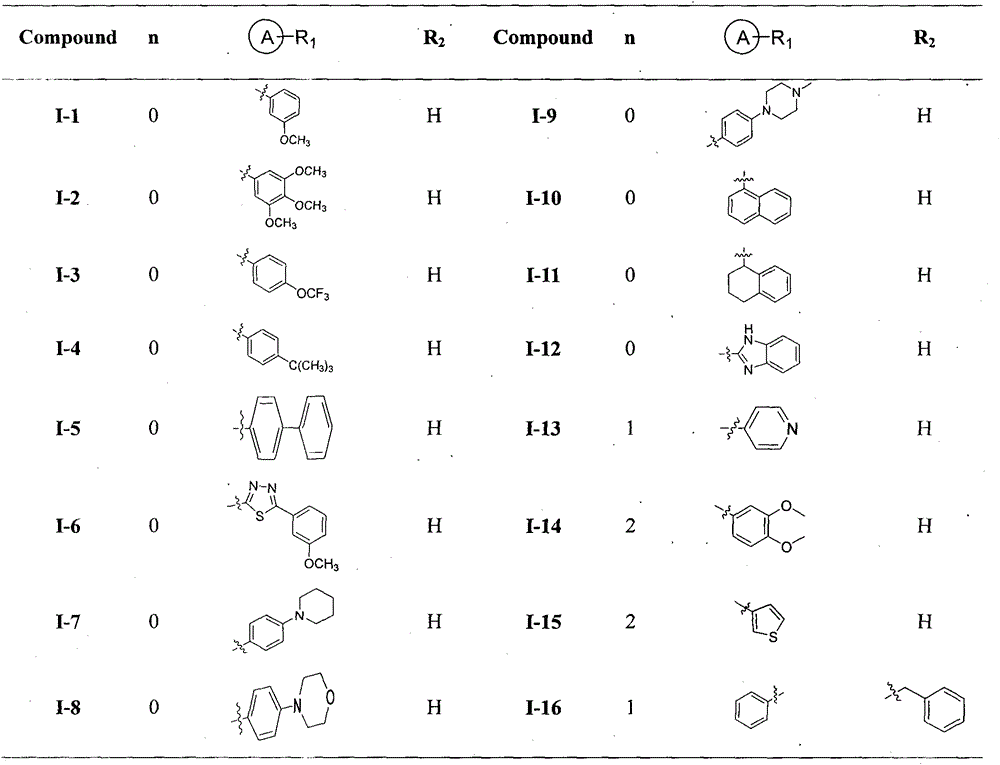
[0089]Preparation of N-(3-(methoxy)phenyl)-2-((pyridine-4-methylene)amino)nicotinamide (I-1)
[0090]
[0091] Compound 3 (6mmol), 3-methoxyaniline (5mmol), EDCI (7.2mmol), HOBT (7.2mmol) were dissolved in DMF, stirred at room temperature for 16h, then added 50ml of water to the reaction solution, ethyl acetate ( 30ml×3) extraction, combined organic phases, washed with saturated sodium carbonate solution (20ml×2) and saturated brine (20ml×2), dried over anhydrous sodium sulfate, filtered, the filtrate was evaporated under reduced pressure to remove the solvent, and the residue was subjected to column chromatography (dichloromethane / methanol, 50:1, v / v), purified to obtain 0.88 g of white solid, yield 53%, melting point: 113-115°C.
[0092] 1 H NMR (300MHz, DMSO-d 6 )δppm: 10.59 (s, 1H, -CONH-), 9.23 (s, 1H, ArH), 8.87 (d, J=5.4Hz, 2H, ArH), 8.41 (d, J=7.0Hz, 1H, ArH) , 8.07(d, J=5.4Hz, 3H, ArH), 7.45(s, 1H, ArH), 7.32(d, J=6.9Hz, 2H, ArH), 6.97(d, J=5.7Hz, 1H, ArH ), 6....
Embodiment 3
[0094] Preparation of 2-((pyridine-4-methylene)amino)-N-(3,4,5-(trimethoxy)phenyl)nicotinamide methanesulfonate (I-2)
[0095]
[0096] Compound I-1 in Preparation Method Example 2 was prepared from compound c (6mmol) 3,4,5-(trimethoxy)aniline (5mmol) to obtain compound I-2 to obtain 1.21g of white solid with a yield of 61%. Melting point: 216-218°C.
[0097] 1 H NMR (300MHz, DMSO-d 6 )δppm: 10.32 (s, 1H, -CONH-), 8.83 (d, J = 6.1Hz, 3H, ArH), 8.20 (d, J = 7.3Hz, 1H, ArH), 8.10 (d, J = 4.1Hz , 1H, ArH), 7.97 (d, J=5.9Hz, 2H, ArH), 7.20 (s, 2H, ArH), 6.85-6.67 (m, 1H, -NH-), 4.94 (s, 2H, -NHC H 2 -), 3.78(s, 6H, -OCH 3 ), 3.65(s, 3H, -OCH 3 ), 2.37(s, 6H, -CH 3 ); 13 C NMR (75MHz, DMSO-d 6 )δ: 162.22, 155.04, 151.12, 149.22, 144.38, 140.57, 137.08, 133.41, 125.26, 116.37, 108.08, 101.24, 60.70, 56.83, 43.29, 38.57, 36.02]; ESI-MS m / z: 3 + ;Anal.calcd.For C 23 h 28 N 4 o 10 S 2 : C, 47.25; H, 4.83; N, 9.58; S, 10.97; Found: C, 47.27; H, 4.70; N, 9.71; S, 10.82....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com