pH-responsive magnetic metal organic framework composite nano-material and its preparation method and use
A composite nanomaterial, magnetic metal technology, applied in the application field of glycoprotein capture and release, can solve problems affecting protein analysis or application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
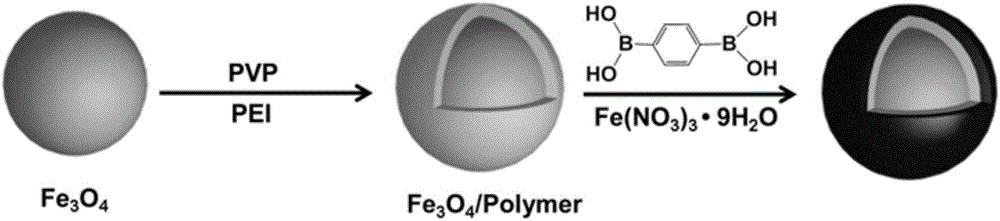
[0044] Embodiment 1 prepares Fe 3 o 4 / Polymer / MOFs Composite Nanomaterials
[0045] In this embodiment, the polymer layer is polyvinylpyrrolidone (PVP), the metal-organic framework uses iron nitrate as a metal source, and 1,4-terephthalenediboronic acid (PBA) as an organic ligand.
[0046] figure 1 gives Fe 3 o 4 / Polymer / MOFs composite nanomaterials preparation flow chart, firstly use the solvothermal method to prepare the surface negatively charged Fe 3 o 4 Nanoparticles; then according to the interaction of electrostatic interaction and van der Waals force, the obtained Fe 3 o 4 The surface of the nanoparticles is coated with a layer of polymer layer, and the Fe coated with the polymer layer is obtained. 3 o 4 Nanospheres (Fe 3 o 4 / Polymer nanoparticles); then ferric nitrate as the metal source, 1,4-terephenylboronic acid (PBA) as the organic ligand, and Fe 3 o 4 / Polymer nanoparticles together to prepare Fe by one-pot method 3 o 4 / Polymer / MOFs composite n...
Embodiment 2
[0056] Embodiment 2 prepares Fe 3 o 4 / Polymer / MOFs Composite Nanomaterials
[0057] In this embodiment, the polymer layer is polyvinylpyrrolidone (PVP) and polyetherimide (PEI), the metal-organic framework uses ferric nitrate as the metal source, and 1,4-terephenylboronic acid (PBA) as the organic ligand. body.
[0058] Fe in this example 3 o 4 / Polymer / MOFs composite nanomaterial preparation steps are as follows:
[0059] (1) Preparation of Fe 3 o 4 Nanoparticles
[0060] Add raw materials 1.157g ferric chloride hexahydrate, 0.4g sodium citrate and 3.303g ammonium acetate to a polytetrafluoroethylene stainless steel reaction kettle filled with 60mL solvent ethylene glycol, and magnetically stir for 1 hour to dissolve the above raw materials; then transfer Remove the stirrer, raise the temperature of the reactor to 200°C, and react for 16 hours; then cool the reactor to room temperature, and collect the product in the reaction solution obtained by the reaction with a ...
Embodiment 3
[0067] Embodiment 3 prepares Fe 3 o 4 / Polymer / MOFs Composite Nanomaterials
[0068] In this embodiment, the polymer layer is polydopamine (PDA) and polyacrylic acid (PAA), the metal-organic framework uses ferric chloride as the metal source, and 3-carboxyphenylboronic acid as the organic ligand.
[0069] The preparation steps of the Fe3O4 / Polymer / MOFs composite nanomaterial in this example are as follows:
[0070] (1) Preparation of Fe 3 o 4 Nanoparticles
[0071] Add raw materials 1.157g ferric chloride hexahydrate, 0.7g sodium citrate and 3.303g ammonium acetate to a polytetrafluoroethylene stainless steel reaction kettle filled with 60mL solvent ethylene glycol, and stir magnetically for 1 hour to dissolve the above raw materials; then transfer Remove the stirring bar, raise the temperature of the reactor to 220°C, and react for 17 hours; then cool the reactor to room temperature, and collect the product in the reaction liquid obtained by the reaction with a magnet; t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com