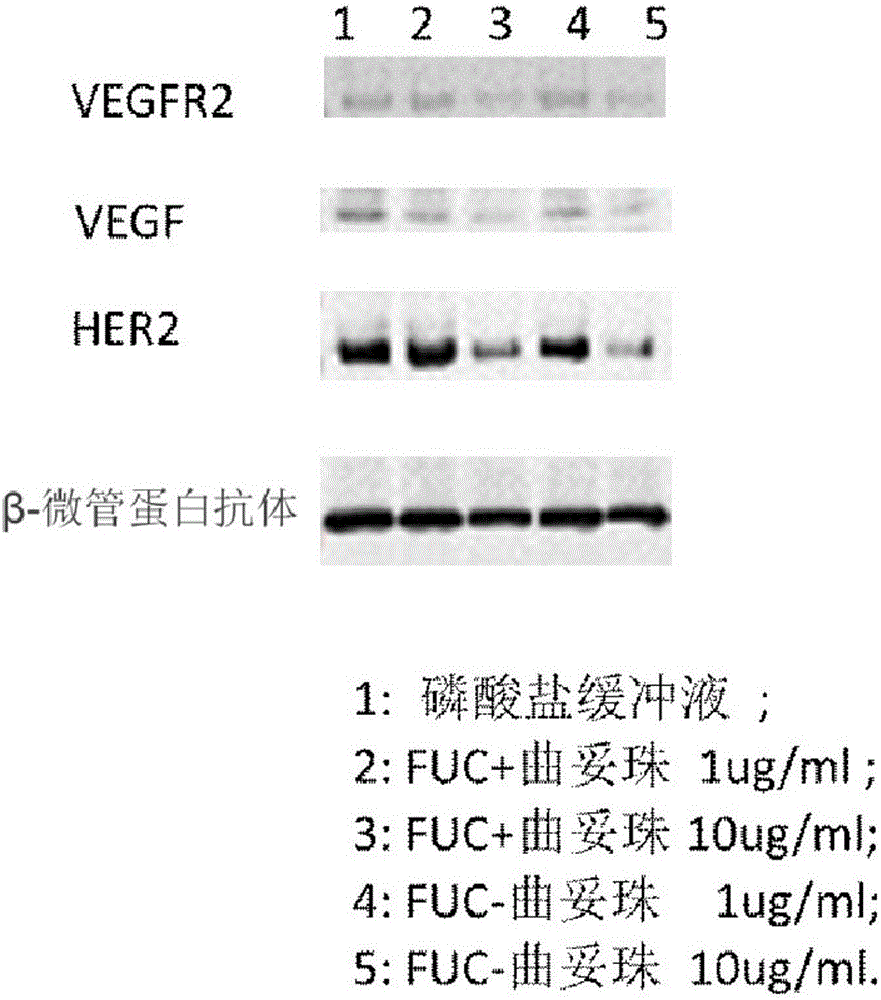
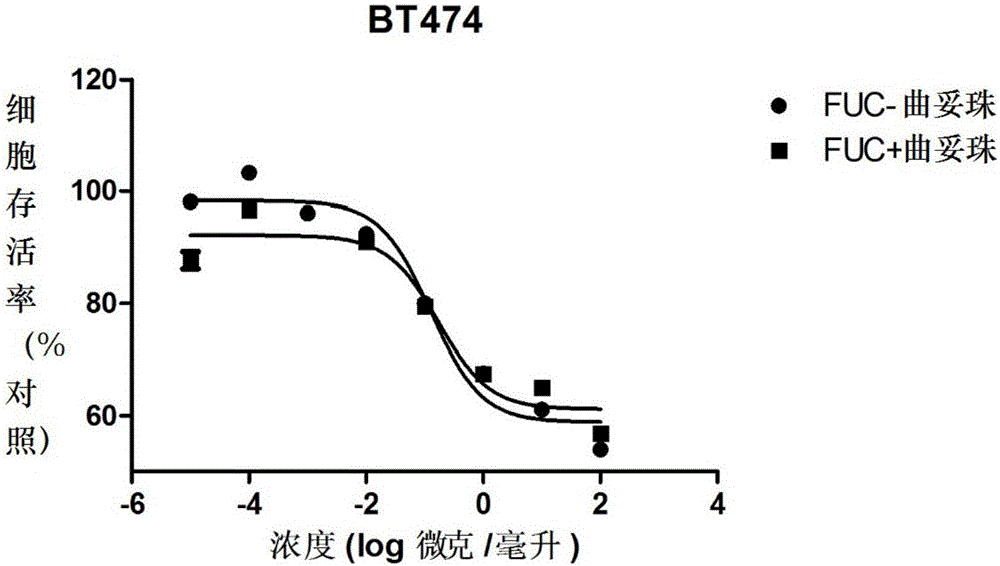
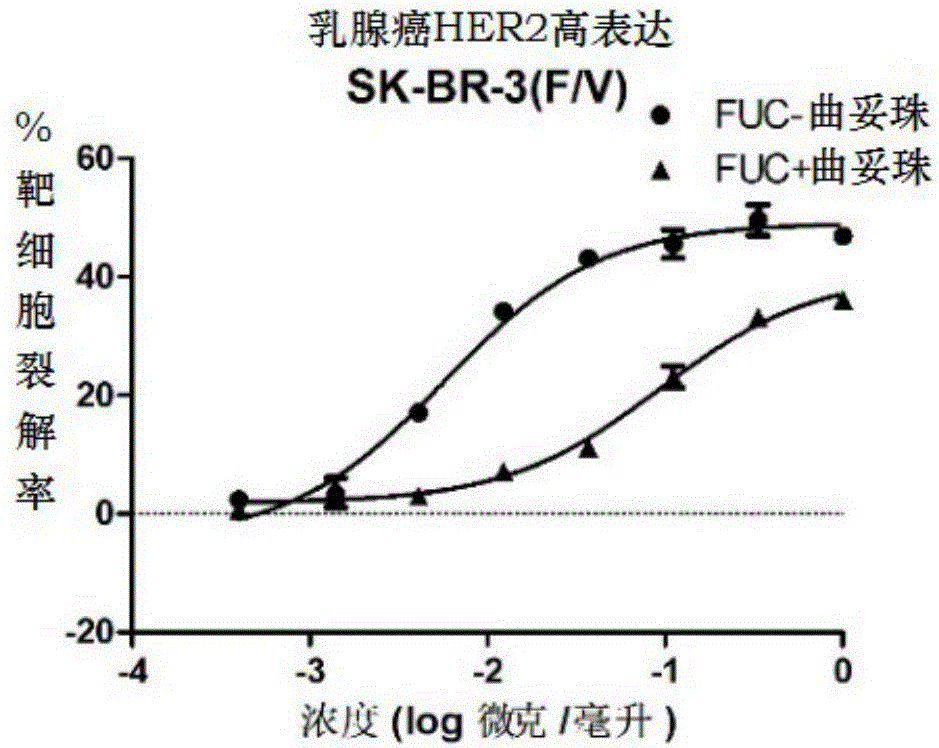
Fucose-removed anti-HER2 antibody and application thereof
A fucose and fucoid-removing technology, applied in the field of fucose-removing anti-HER2 antibodies, can solve the problem that patients cannot benefit from Herceptin, and achieve inhibition of tumor angiogenesis, high ADCC activity, and the probability of drug resistance small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: Expression and purification of antibodies
[0077] The antibody light and heavy chain sequences were cloned into mammalian expression vectors using the human growth factor 1 (hEF) promoter. The expression vector was transfected into CHO-S cells or FUT-8 knockout CHO-S cells, the cells were grown in Freestyle Max reagent (purchased from Invitrogen) medium, and stable expression strains were screened with puromycin. The stable cell line was cultured in a 1-liter shake flask, and the supernatant was collected after 14 days, and the antibody was purified using a protein A column. After the antibody was eluted from the column, it was dialyzed with PBS to obtain the purified antibody. If the antibody is from CHO-S cells, a high-fucose anti-HER2 antibody will be obtained; if the antibody is from a FUT-8 gene knockout CHO-S cell, a fucose-free anti-HER2 antibody will be obtained. There are three kinds of DUXB11, DG44 and CHOK1 in CHO cells, among which CHOK1 is wild...
Embodiment 2
[0078] Example 2: Comparison of fucose content in fucose-free anti-HER2 antibody and high-fucose anti-HER2 antibody.
[0079] Antibody glycans were purified from 100 μg of antibody after digestion with trypsin and glycopeptidase, followed by mass spectrometry analysis. The identification of each glycan depends on its mass-to-charge ratio m / z, and all detected glycan concentrations are derived from comparison with an internal reference.
[0080] Glycosyl nomenclature
[0081]
[0082] ◆ N-acetylneuraminic acid N-acetylgalactosamine glucose
[0083] Galactose N-Glucosamine N-glycolylgalactosamine
[0085] Glycosyl number definition: 5 numbers respectively express the number of different sugars, six-carbon sugar (Galactose, Mannose, or Glucose), N-acetylhexosamine (GlcNAc or GalNAc), fucose (Fucose, FUC for short), N-acetylneuraminic acid (Neu5Ac), and N-glycolylneuraminic acid (Neu5Gc). The third number represents whether fucose i...
Embodiment 3
[0096] Example 3: Comparison of the affinities of the anti-HER2 antibody with no fucose and the anti-HER2 antibody with high fucose to the ECD of the extracellular region of HER2.
[0097] The experiments were performed on a Biacore T200. The mobile phase is PBS buffer (NaCl 8.0g, KCl 0.2g, KH 2 PO 4 0.24g, Na 2 HPO 4 ×12H 2 O 3.628g, be dissolved in 800ml distilled water, adjust pH value to be 7.4 with hydrochloric acid, distilled water is settled to 1000ml), all samples are all dissolved in the above-mentioned PBS damping fluid.
[0098] The fucose-free anti-HER2 antibody and the high-fucose anti-HER2 antibody were immobilized on the CM5 sensor chip through amino coupling, and the flow rate of the flow cell was set to 10ul / min, and the concentration of 0.1mol / L 1-B The surface of the CM5 chip was activated with an equal volume mixed solution of 3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) and 0.1mol / L N-N-hydroxysuccinimide (NHS). Inject 50mg / ml of fucose-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com