A method for regenerating circulating working fluid in the process of producing hydrogen peroxide by anthraquinone method and a method for producing hydrogen peroxide
A technology of hydrogen peroxide and circulating work, applied in the direction of peroxide/peroxy hydrate/peroxyacid/superoxide/ozonide, chemical instruments and methods, chemical recovery, etc., can solve the regeneration efficiency of circulating working fluid Low-level problems, to achieve the effect of improving recycling efficiency, reducing cumulative content, and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
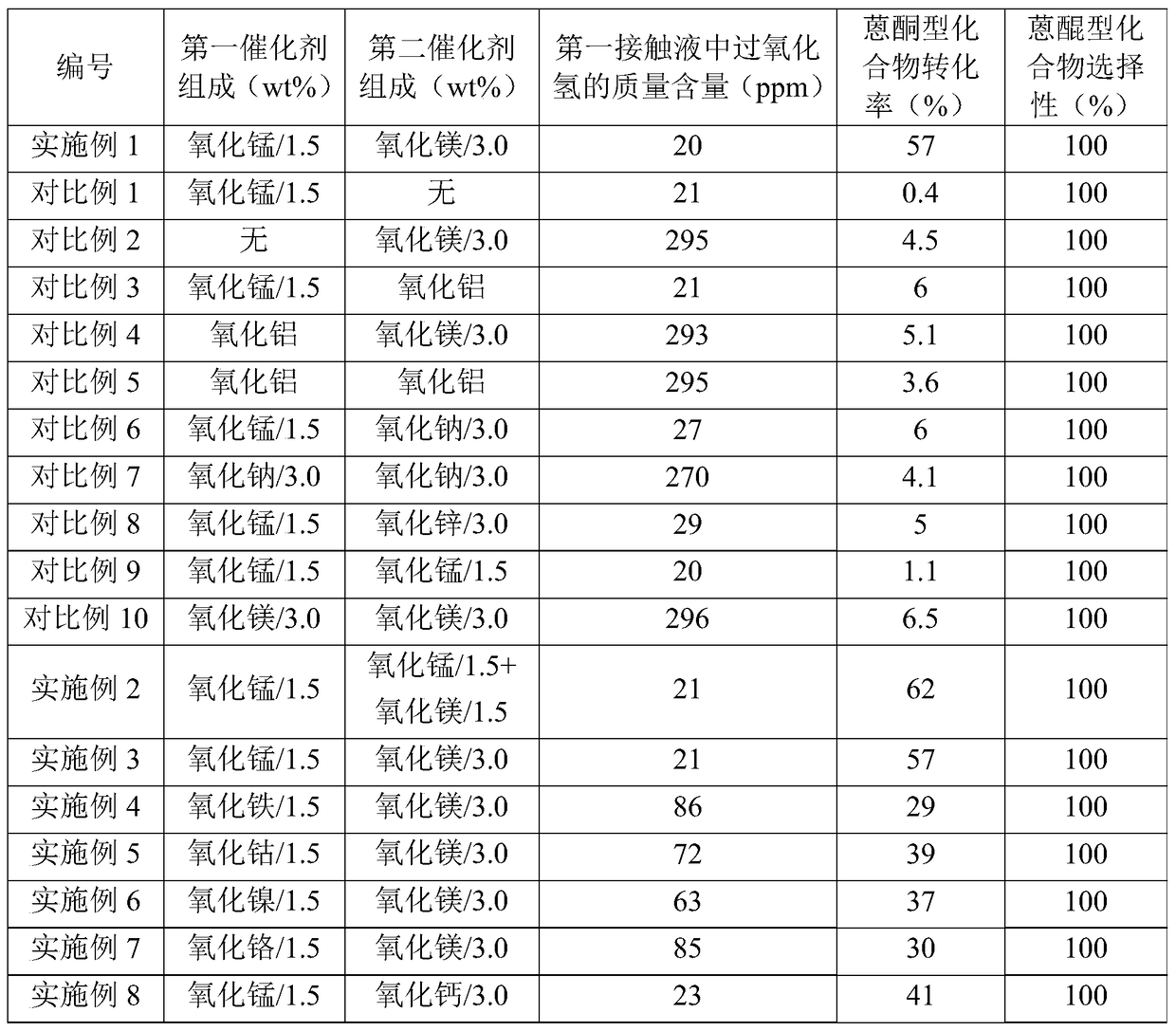
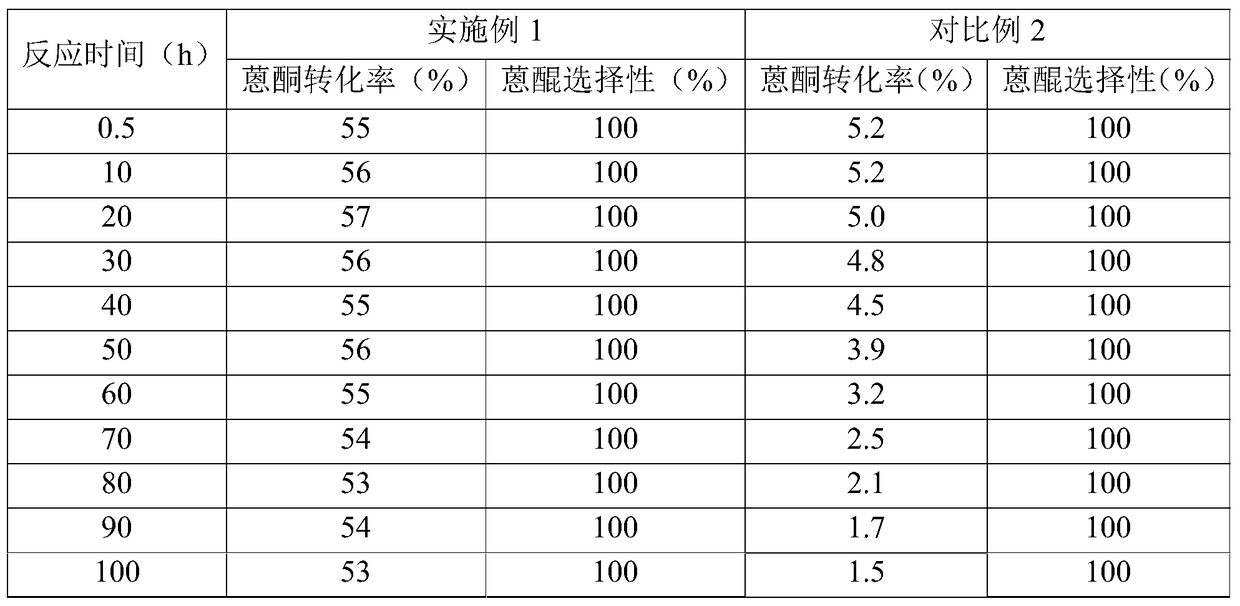
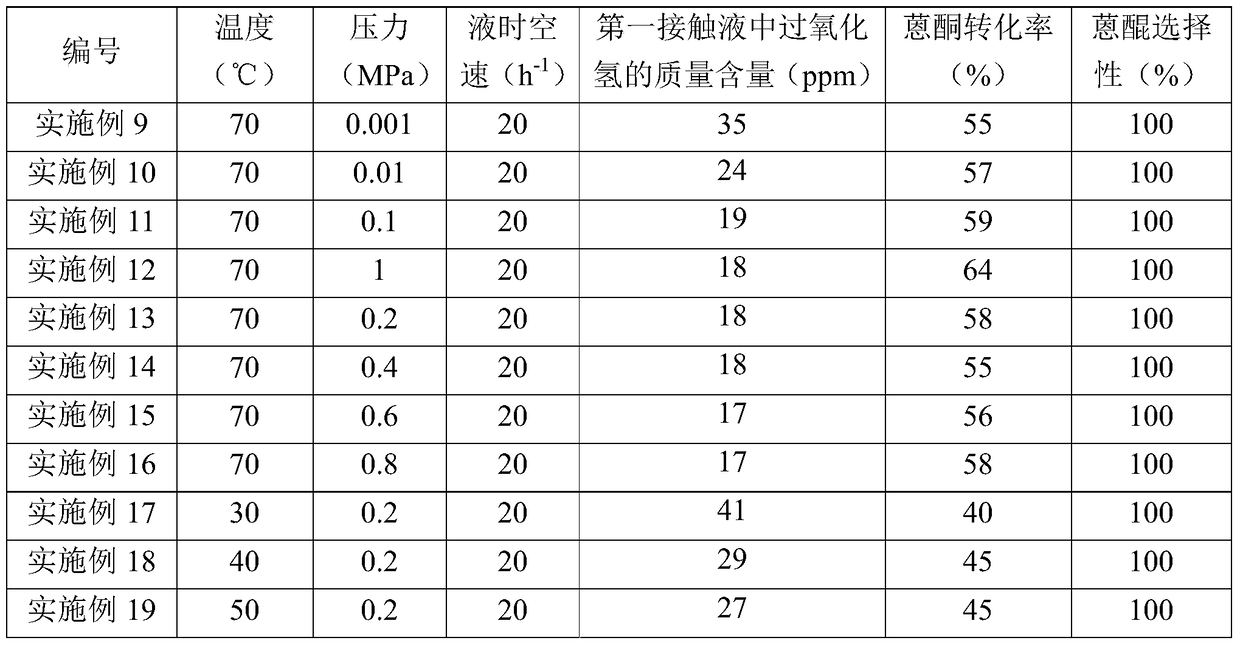
Embodiment 1
[0089] (1) Preparation of the first catalyst
[0090] Mn(NO 3 ) 2 Dissolve in water to prepare 50mL impregnation solution. In the immersion solution, Mn(NO 3 ) 2 The content is 2g. 50g of alumina (purchased from Bailingwei, product number 13-0750) was added to the above impregnation solution, and saturated with stirring at a temperature of 60°C for 8h. Then, the impregnated mixture was dried under normal pressure at 130° C. for 4 hours, followed by calcination at 600° C. for 4 hours, thereby obtaining the first catalyst according to the present invention (the composition is listed in Table 1).
[0091] (2) Preparation of the second catalyst
[0092] Mg(NO 3 ) 2 Dissolve in water to prepare 50mL impregnation solution. In the immersion solution, Mg(NO 3 ) 2 The content is 5.6g. 50g of alumina (purchased from Bailingwei, product number 13-0750) was added to the above impregnation solution, and saturated with stirring at a temperature of 60°C for 8h. Then, the impregn...
Embodiment 2
[0118] The difference between this embodiment and Example 1 is that the catalyst packed in the second miniature fixed-bed reactor is prepared by the following method: Mn(NO 3 ) 2 and Mg(NO 3 ) 2 Dissolve in water to prepare 50mL impregnation solution. In the immersion solution, Mn(NO 3 ) 2 The content is 1.9g, Mg(NO 3 ) 2 The content is 2.8g. 50g of alumina (purchased from Bailingwei, product number 13-0750) was added to the above impregnation solution, and saturated with stirring at a temperature of 60°C for 8h. Then, the impregnated mixture was dried under normal pressure at 130° C. for 4 hours, followed by calcining at 600° C. for 4 hours, thereby obtaining the second catalyst according to the present invention (the composition is listed in Table 1). The experimental results are listed in Table 1.
Embodiment 3
[0120] The difference between this embodiment and Example 1 is that the reaction conditions of the first miniature fixed-bed reactor are different from Example 1 (but the reaction conditions of the second miniature fixed-bed reactor are identical with Example 1): the first miniature fixed-bed The temperature in the middle catalyst bed was controlled at 60°C. The experimental results are listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com