Codon-optimized Chi3L1 gene and application thereof
A codon optimization and gene technology, applied in the field of genetic engineering, can solve the problems of low biological activity and low expression of Chi3L1, and achieve the effects of strong immune activity, good foundation and clinical application prospects, and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Construction of recombinant Chi3L1 protein expression plasmid.
[0029] Synthesize the Chi3L1 gene (as shown in ESQ ID No.: 1) first, and then subclone it into the pRSET-A plasmid by using NdeI and HindIII restriction sites to obtain the pRSET-A-Chi3L1 expression plasmid. The specific construction process as follows:
[0030] (1). Enzyme digestion: the synthesized DNA fragment of Chi3L1 was digested with restriction endonucleases NheI and HindIII, and the pRSET-A empty shuttle plasmid was digested with corresponding endonucleases.
[0031] Table 1 enzyme digestion reaction system
[0032]
[0033] Reaction conditions: 37°C water bath for 2h. The above digested products were separated by 1% agarose gel electrophoresis to recover the target fragment, and the purified target DNA fragment was obtained using Agarose Gel DNA Extraction Kit.
[0034] (2). Ligation: Ligate the expression fragment of Chi3L1 digested with the pRSET-A vector.
[0035] Table 3 Con...
Embodiment 2
[0038] Example 2: This example describes the expression and purification method of recombinant Chi3L1 protein
[0039] The expression plasmid pRSET-A has a continuous encoding of 6 histidines (His 6 ) base sequence, therefore, the expressed polypeptide has His at the amino terminal 6 Marker for easy purification and identification of expressed proteins. The expression plasmid was transformed into BL21 competent bacteria. After overnight culture, shake culture in 100ml SOB medium at 1:50 at 37°C. When the OD450 was about 0.5, add IPTG with a final concentration of 0.5mM and continue to culture for 4h; , 4h respectively sample 1ml, centrifuge at 6000r / min for 5min, add 30μl Laemmli buffer solution to the precipitate, heat at 95°C for 5min, take 8μl by SDS-PAGE, Coomassie brilliant blue staining analysis shows that with the prolongation of induction time, at the position of relative molecular mass 42kD The nearby protein bands were gradually enhanced, and the protein expression...
Embodiment 3
[0041] Example 3: Recombinant Chi3L1 protein promotes macrophage M2 differentiation
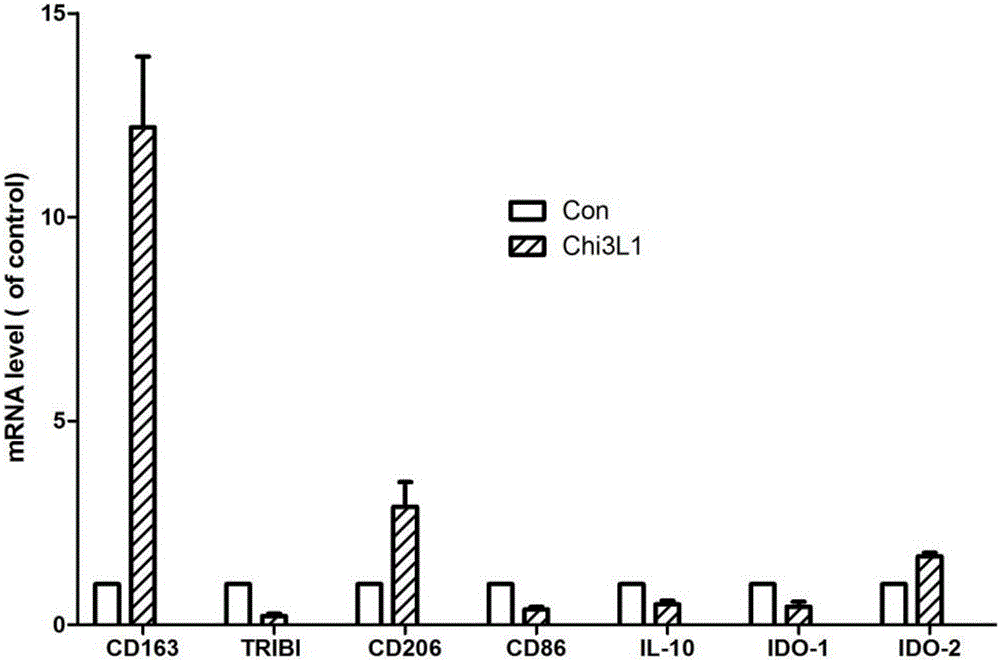
[0042]Macrophages were treated with 1000 μg / ml purified Chi3L1 protein for 4 hours, the cells were collected, RNA was extracted, and the expression levels of macrophage differentiation-related genes were detected by RT-PCR after reverse transcription. The results showed that CD163, CD206 and other macrophage M2 The expression of differentiation-related genes was significantly increased, suggesting that the Chi3L1 recombinant protein can effectively promote the M2 differentiation of macrophages ( figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com