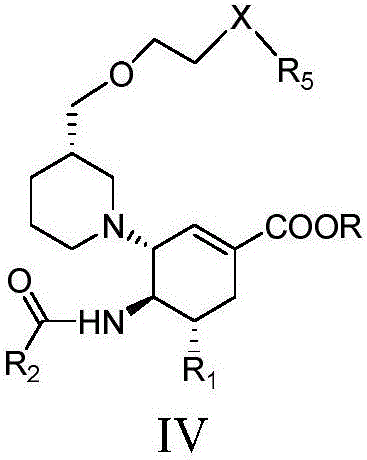
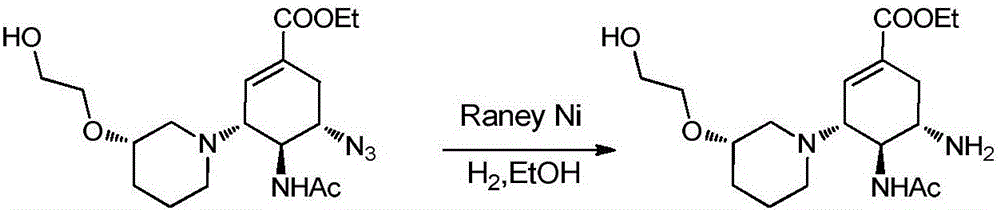
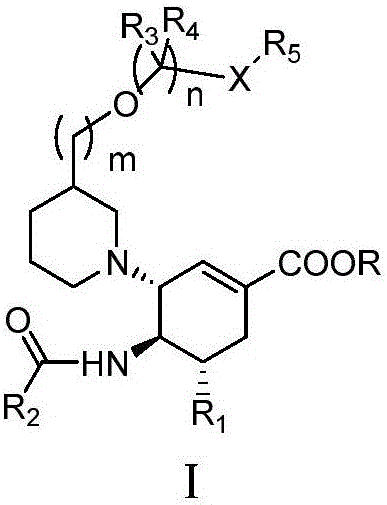
Cyclohexene compounds and application thereof
A compound, cyclohexene technology, applied in the directions of organic chemistry, drug combination, medical preparations containing active ingredients, etc. Superior application prospects, the effect of inhibiting influenza virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] a) (3R, 4S, 5S)-4-acetylamino-5-azido-3-((3S)-3-(2-methoxyethoxy)piperidine)-1-cyclohexene-1 - Preparation of ethyl formate
[0059]
[0060] According to the above reaction formula, 20 mmol of (3R, 4R, 5S)-4-acetylamino-5-azido-3-acetoxy-1-cyclohexene-1-carboxylic acid ethyl ester and 1 mmol of tetratriphenyl The phosphine palladium was placed in a dry two-necked bottle, and after the air in the system was replaced twice with nitrogen, 40 mL of redistilled DMF (dimethylformamide) was added with a syringe and stirred evenly, and 40 mmol of DIPEA (N, N-dimethylformamide) was added. isopropylethylamine) and stirred and cooled to 0°C, slowly added dropwise a solution of (3S)-3-(2-methoxyethoxy)piperidine trifluoroacetate in DMF (40mL), and continued Stir at 0°C for 20 minutes, move to an oil bath at 70°C for 1 hour, thin layer chromatography TLC (DCM:MeOH=10:1, i.e. dichloromethane:methanol=10:1) shows that the reaction is complete, slowly cool to 0°C, slowly Slowly a...
Embodiment 2
[0082] a) (3R, 4S, 5S)-4-acetylamino-5-azido-3-((3R)-3-(2-methoxyethoxy)piperidine)-1-cyclohexene-1 - Preparation of ethyl formate
[0083]
[0084] (3R,4S,5S)-4-Acetamido-5-azido-3-((3R)-3-(2-methoxyethoxy)piperidine)-1-cyclohexene-1-carboxylic acid Ethyl ester was prepared with reference to Example 1a).
[0085] Product characterization: 1 H-NMR (400MHz, CDCl 3 ): δppm6.837 (s, 1H, N H ), 5.715-5.694 (dd, 1H, 2-C H ), 4.201-4.184 (q, 2H, C H 2 CH 3 ), 3.925-3.877 (dd, 1H, 4-C H ), 3.781-3.715 (dd, 1H, 3-C H ), 3.604-3.530 (t, 2H, OC H 2 CH 2 O), 3.502-3.491 (m, 1H, NCH 2 C H OCH 2 ), 3.490-3.451 (t, 2H, OCH 2 C H 2 O), 3.351(s, 3H, OC H 3 ), 3.282-3.239 (d, 1H, NC H 2 CHOCH 2 ), 3.016-2.991 (t, 1H, NC H 2 CH 2 ), 2.893-2.837 (t, 1H, NC H 2 CH 2 ), 2.529-2.516 (m, 2H, NC H 2 CHOCH 2 , 5-C H ), 2.352-2.285 (dd, 1H, 6-C H 2 ), 2.248-2.202 (m, 1H, NCH 2 CH 2 C H 2 ), 2.182-2.177 (dd, 1H, 6-C H 2 ), 2.061(s, 3H, COC H 3 ), 1.959-1.935...
Embodiment 3
[0106] a) (3R, 4S, 5S)-4-acetylamino-5-azido-3-((3S)-3-((2-hydroxyethoxy)piperidine)-1-cyclohexene-1- Preparation of ethyl formate
[0107]
[0108] (3R, 4S, 5S)-4-acetylamino-5-azido-3-((3S)-3-((2-hydroxyethoxy)piperidine)-1-cyclohexene-1-carboxylic acid ethyl Esters were prepared with reference to Example 1a).
[0109] Product characterization: 1 H-NMR (400MHz, CDCl 3 ): δppm6.869 (s, 1H, N H ), 5.561-5.539 (dd, 1H, 2-C H ), 4.255-4.193 (q, 2H, COOC H 2 CH 3 ), 4.014-3.940 (dd, 1H, 4-C H ), 3.834-3.193 (m, 2H, OCH 2 C H 2 OH), 3.702-3.635 (m, 2H, OC H 2 CH 2 OH), 3.419-3.395 (dd, 1H, 3-C H ), 2.958-2.943 (m, 1H, NCH 2 C H ), 2.914-2.900 (m, 1H, NC H 2 CH), 2.787-2.750 (m, 1H, NC H 2 CH 2 ), 2.711-2.661 (m, 1H, NC H 2 CH 2 ), 2.585-2.261 (m, 1H, NC H 2 CH), 2.554-2.534 (m, 1H, 5-C H ), 2.332-2.262 (m, 1H, 6-C H 2 ), 2.071(s, 3H, COC H 3 ), 1.846-1.811 (m, 2H, NCH 2 C H 2 C H 2 ), 1.507-1.466 (m, 2H, NCH 2 C H 2 C H 2 ), 1.329-1.295...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com