New cath2 derivatives
A derivative, CMAP27-technology, applied to CATH2. , the peptide domain of CMAP27
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] Some broader summaries of methods that can be applied in the preparation of peptides are described in: W.F. Anderson, Nature 392 Supp., April 30, 1998, p. 25-30; Pharmaceutical Biotechnology, Ed.D.J.A. Crommelin and R.D. Sindelar , Harwood Academic Press, 1997, p.53-70, 167-180, 123-152, 8-20; Protein Synthesis: Methods and Protocols, Ed.R. Martin, Humana Press, 1998, p.1-442; Solid-Phase Peptide Synthesis, Ed.G.B. Fields, Academic Press, 1997, p.1-780; Amino Acid and Peptide Synthesis, Oxford University Press, 1997, p.1-89.
[0043] Novel peptides as disclosed herein can be readily prepared by those skilled in the art.
[0044] The CMAP27- or CATH2-derivatives of the present invention can be used alone or in combination in the form of a multimer. Suitable combinations of the peptides of the invention include concatemers of the peptides of the invention sequentially linked to each other by spacers, e.g., in the form of peptide dimers, peptide trimers, etc., wherein the...
Embodiment 1
[0077] Example 1 - Efficacy of CMAP-27 derivative peptides administered in ovo
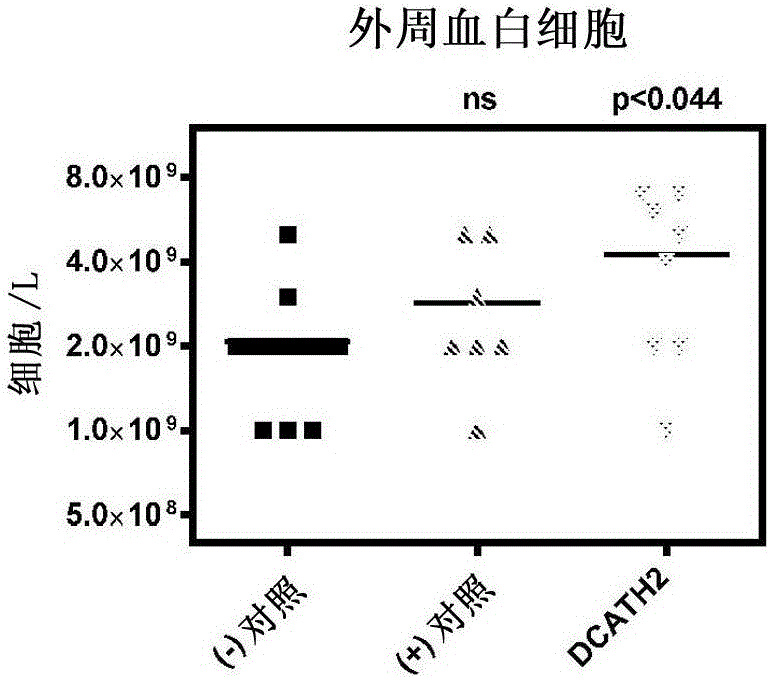
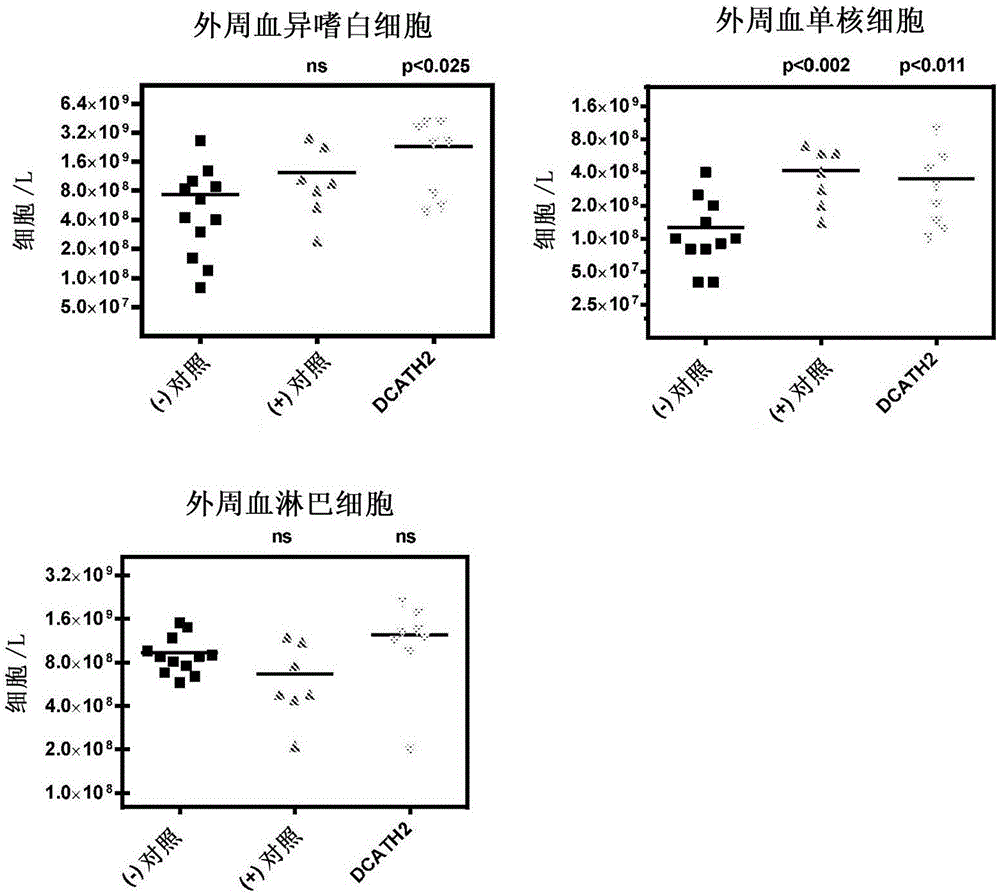
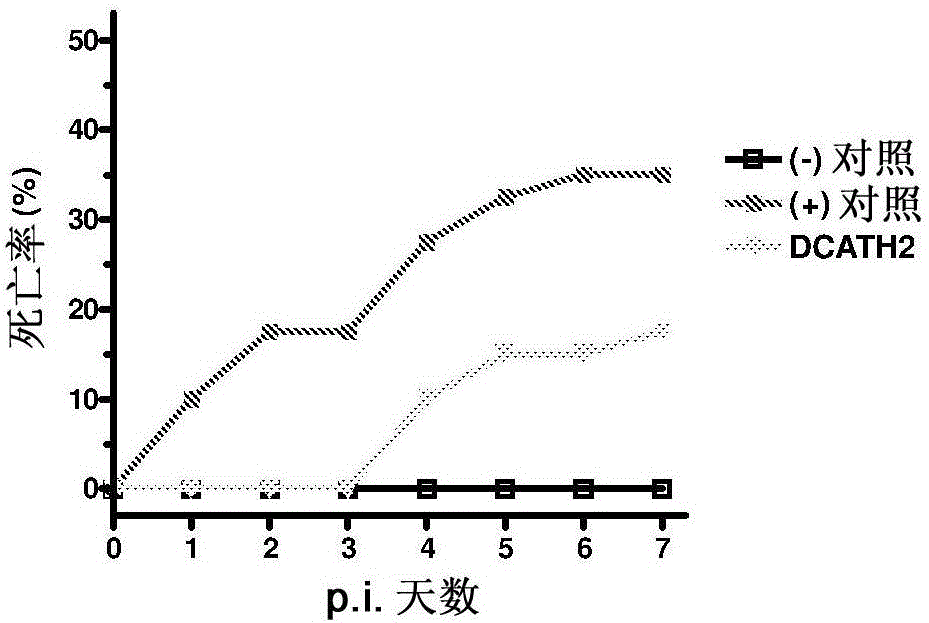
[0078] DCATH2 peptide (complete D-amino acid analog of CMAP1-26) was suspended in cholesterol / PBS formulation. At the 18th day of the embryonic period, 100 μl of DCATH2-containing suspension was used to inject Rose 308 eggs in the amniotic fluid, and then returned to the incubator for incubation (at the embryonic day of about 21 days). Two control groups were injected in the amniotic fluid with the cholesterol / PBS formulation. Peptide doses of 1 mg / kg body weight were calculated based on an embryo weight of 22 g. After hatching, the birds were transferred to 4 pens per treatment (n=40 / group). 3 days after hatching, in addition to the negative control, 5 × 10 6 Dose of CFU / bird, all birds were inoculated subcutaneously with the avian pathogenic Salmonella enteritidis pt13a strain. Two days after the challenge, 12 birds per group were sacrificed and samples were collected for blood and organ ana...
Embodiment 2
[0083] Example 2 - Prevention of E. coli infection following in ovo administration of peptides.
[0084] DCATH2 peptide was suspended in cholesterol / PBS formulation. At the 18th day of the embryonic period, 100 μl of DCATH2-containing suspension was used to inject Rose 308 eggs in the amniotic fluid, and then returned to the incubator for incubation (at the embryonic day of about 21 days). Two control groups were injected in the amniotic fluid with the cholesterol / PBS formulation. Based on an embryo weight of 22 g, a DCATH2 peptide dose of 1 mg / kg body weight was calculated. After hatching, the birds were transferred to each treatment isolation unit (n=54 / group). 3 days after hatching, in addition to the negative control, 1 × 10 6 The dose of CFU / bird, all birds were inoculated with avian pathogenic Escherichia coli (Escherichia coli) 506 strain (UU strain) in the trachea. Two days after challenge, 13 birds per group were sacrificed and used to collect samples for blood an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com