Near-infrared aza-BODIPY dye and microwave synthesis method thereof
A fluorine boron dipyrrole and near-infrared technology, which is applied to azo dyes, organic dyes, chemical instruments and methods, and can solve problems such as low yield, long reaction time, and long completion time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]Synthesis of near-infrared azafluoroboron dipyrrole dye compound 4:
[0041] 1. Synthesis of compound 1:
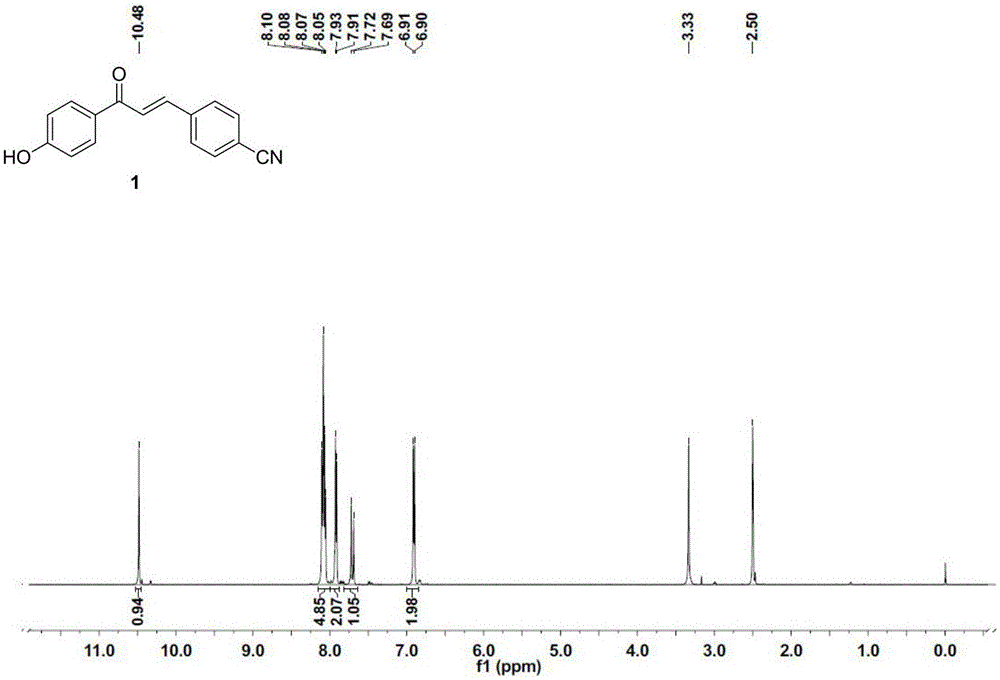
[0042] p-Hydroxyacetophenone (1.36g, 10mmol) and p-cyanobenzaldehyde (1.31g, 10mmol) were dissolved in 50mL of absolute ethanol, KOH (560mg, 30mmol) was added, stirred overnight at room temperature, and then KOH (560mg, 30mmol) was added , stirred at room temperature for 5h, suction filtered, the filtrate was adjusted to pH=4.0 with 3mol / L hydrochloric acid, precipitated, filtered with suction, and the filter cake was washed with 3mol / L hydrochloric acid to obtain compound 1 as light yellow solid powder (1.80g, yield 72.3%). 1 H NMR (500MHz, DMSO-d 6 )δ10.48(s,1H),8.10-8.05(m,5H),7.92(d,J=5.0Hz,2H),7.72-7.69(d,J=15.0Hz,1H),6.91-6.90(d ,J=5.0Hz,2H). 13 C NMR (126MHz, DMSO-d 6 )δ186.89,162.48,140.46,139.48,132.65,131.38,129.24,128.80,118.65,115.44,112.01.HRMS(ESI+)calcd forC 16 h 11 NO 2 [M+H] + 250.0863, found 250.0848.
[0043] 2. Synthesis of compound 2: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com