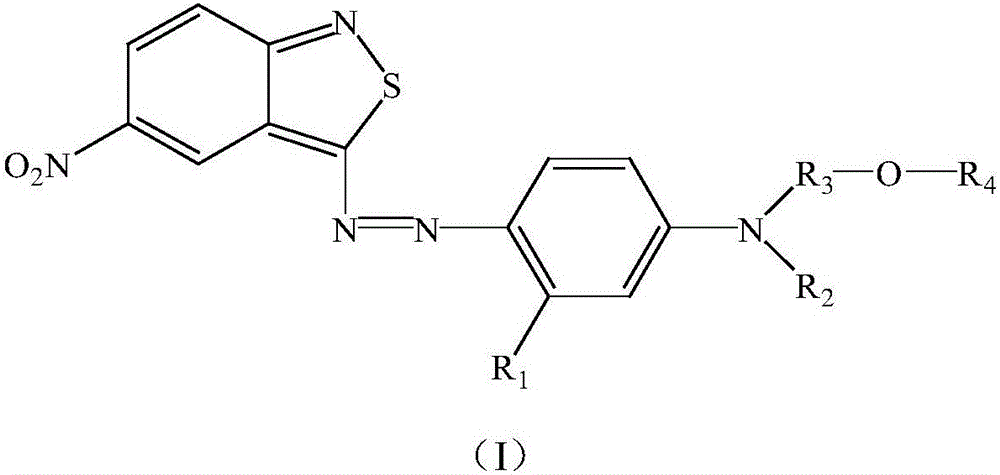
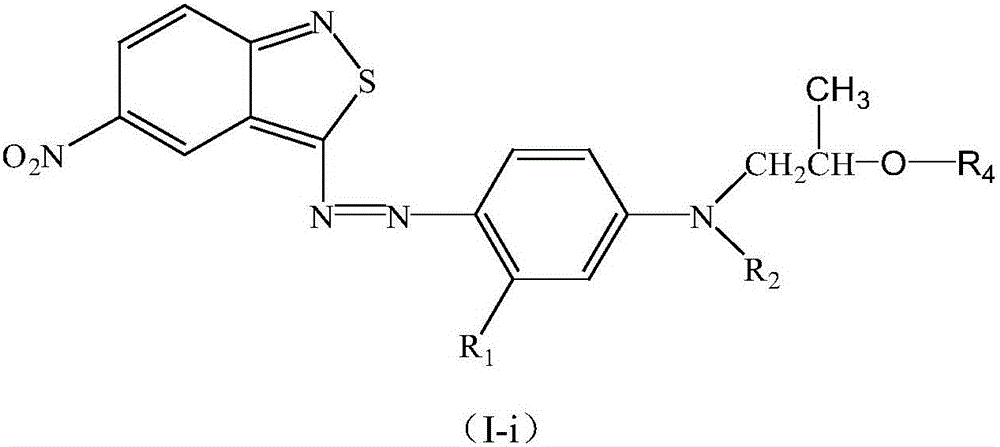
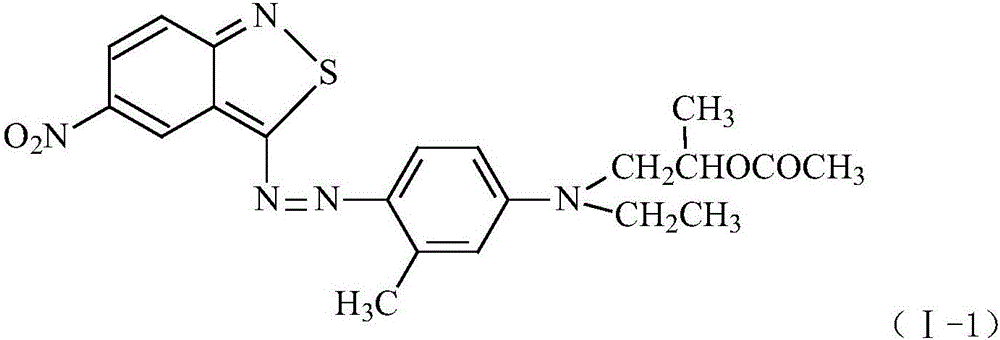
Benzisothiazole dye monomer compound, and intermediates, preparation method and application thereof
A technology of benzisothiazole and dye monomers, which is applied in the preparation of organic compounds, chemical instruments and methods, dyeing methods, etc., can solve the problems of difficult to achieve, poor comprehensive performance, etc., and achieve simple synthesis process, less discharge of three wastes, Inexpensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Add 40g of 98% sulfuric acid and 58.5g of 28% nitrosylsulfuric acid into the three-necked flask, stir for 20min, lower the temperature to -5~0°C, and slowly add 18.3g of 3-amino-5-nitro- For 2,1-benzisothiazole, the feeding time is 1 to 2 hours, and the heat preservation reaction is about 4 hours to obtain the diazonium solution of 3-amino-5-nitro-2,1-benzisothiazole.
[0068] Put 135g (1mol) of N-ethyl-m-toluidine into the high-pressure reactor, raise the temperature to 110-120°C and start feeding 60g of propylene oxide for about 4-5 hours, and keep warm for 10-20 hours after passing through , after the heat preservation is completed, the temperature is lowered to 30-40° C. to obtain N-ethyl-N-hydroxyisopropyl-m-toluidine.
[0069] Take the above-mentioned N-ethyl-N-hydroxyisopropyl-m-toluidine, put it into a three-necked flask, raise the temperature to 60°C-100°C, slowly add 62g of glacial acetic acid or 105g of acetic anhydride, keep warm until the end of the reactio...
Embodiment 2
[0073] According to the preparation method described in Example 1, the difference is that N-ethyl-N-acetoxyisopropyl m-toluidine is used in an equimolar amount of N-cyanoethyl-N-acetoxyisopropyl m-toluidine Alternatively, a dye monomer compound represented by structural formula (I-2) can be prepared.
[0074]
[0075] Wherein the preparation method of N-ethyl-N-acetoxy isopropyl m-toluidine is as follows:
[0076] Put 160g (1mol) of N-cyanoethyl m-toluidine into the high-pressure reactor, raise the temperature to 110-120°C and start feeding 60g of propylene oxide for about 4-5 hours, and keep warm for 10-20 hours after passing through. Hours, after the heat preservation is over, lower the temperature to 30-40°C to obtain N-cyanoethyl-N-hydroxyisopropyl-m-toluidine.
[0077] Take the above-mentioned N-cyanoethyl-N-hydroxyisopropyl-m-toluidine, put it into a three-necked flask, raise the temperature to 60°C-100°C, slowly add 62g of glacial acetic acid or 105g of acetic anhyd...
Embodiment 3
[0079] According to the preparation method described in Example 1, the difference is that N-ethyl-N-acetoxyisopropyl m-toluidine is used in an equimolar amount of N-methoxyethyl-N-acetoxyisopropyl m-toluidine Instead of toluidine, the dye monomer compound represented by structural formula (I-3) can be obtained.
[0080]
[0081] The preparation of N-methoxyethyl-N-acetoxyisopropyl-m-toluidine uses N-methoxyethyl-m-toluidine as raw material, and is prepared according to the method in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com