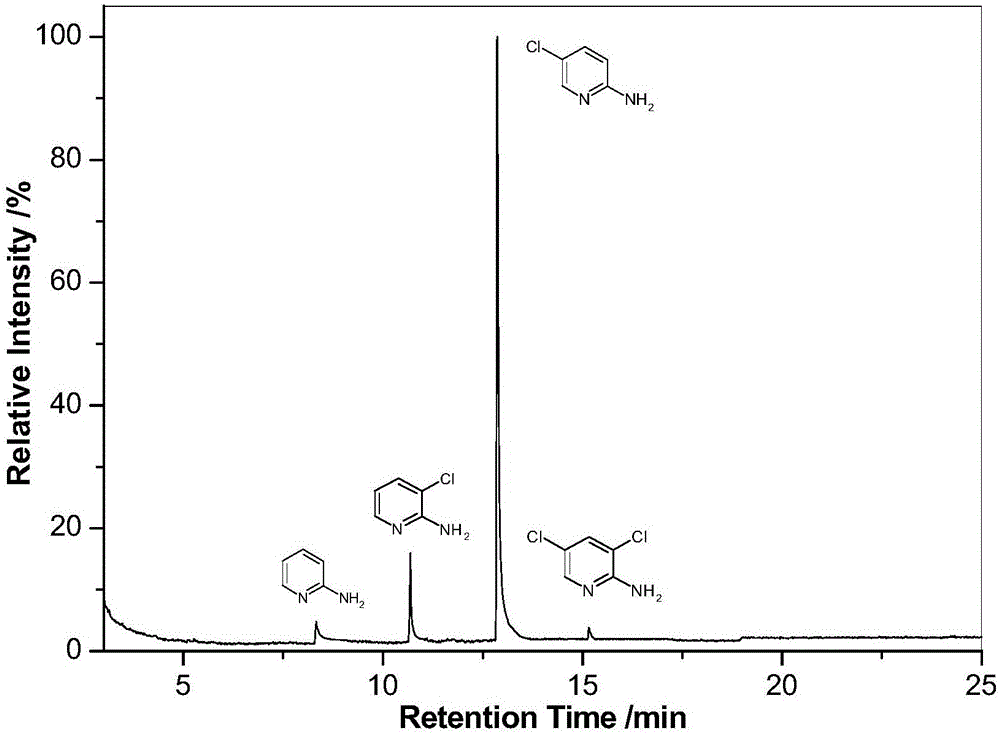
A method for preparation of 2-amino-5-chloro-pyridine
A technology of aminopyridine and pyridine, which is applied in the field of fine organic synthesis to achieve the effects of less pollution, low cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 250ml three-necked flask, add 0.053mol (5.00g) of 2-aminopyridine, and place it in a water bath at 10°C. Under continuous stirring, after adding 0.11 mol of 13% (mass concentration, the same below) NaClO solution, 0.3 mol of 36% (mass concentration, the same below) hydrochloric acid was slowly added dropwise. After reacting at a constant temperature of 10°C for 2h, the temperature was raised to 25°C to continue the reaction for 4h. Finally, the reaction was terminated by cooling down to 10°C with ice water.
[0022] Adjust the above reaction solution to pH>8 with 5mol / L NaOH solution, filter and separate, wash with deionized water, and extract the filtrate and washing liquid with dichloroethane solvent to recover 2-aminopyridine and its chloride. The solid precipitate obtained by filtration and the dichloroethane extract were combined, dissolved in 10% dilute hydrochloric acid, and filtered. The filtrate was adjusted to pH=4 with 5 mol / L NaOH solution, extracted ...
Embodiment 2
[0024] In a 250ml three-necked flask, add 0.053mol (5.00g) of 2-aminopyridine, and place it in a water bath at 10°C. Under continuous stirring, after adding 0.16 mol of 8% NaClO solution, 0.3 mol of 25% hydrochloric acid was slowly added dropwise. After reacting at a constant temperature of 10°C for 2h, the temperature was raised to 25°C to continue the reaction for 4h. Finally, the reaction was terminated by cooling down to 10°C with ice water.
[0025] Adjust the above reaction solution to pH>8 with 5mol / L NaOH solution, filter and separate, wash with deionized water, and extract the filtrate and washing liquid with dichloroethane solvent to recover 2-aminopyridine and its chloride. The solid precipitate obtained by filtration and the dichloroethane extract were combined, dissolved in 10% dilute hydrochloric acid, and filtered. The filtrate was adjusted to pH=4 with 5 mol / L NaOH solution, extracted with dichloroethane solvent, and 5.75 g of the reaction product was obtaine...
Embodiment 3
[0027] In a 250ml three-necked flask, add 0.053mol (5.00g) of 2-aminopyridine, and place it in a water bath at 10°C. Under continuous stirring, after adding 0.11 mol of 13% NaClO solution, 0.25 mol of 36% hydrochloric acid was slowly added dropwise. After reacting at a constant temperature of 10°C for 2h, the temperature was raised to 25°C to continue the reaction for 4h. Finally, the reaction was terminated by cooling down to 10°C with ice water.
[0028] Adjust the above reaction solution to pH>8 with 5mol / L NaOH solution, filter and separate, wash with deionized water, and extract the filtrate and washing liquid with dichloroethane solvent to recover 2-aminopyridine and its chloride. The solid precipitate obtained by filtration and the dichloroethane extract were combined, dissolved in 10% dilute hydrochloric acid, and filtered. The filtrate was adjusted to pH=4 with 5 mol / L NaOH solution, extracted with dichloroethane solvent, and 5.58 g of the reaction product was obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com