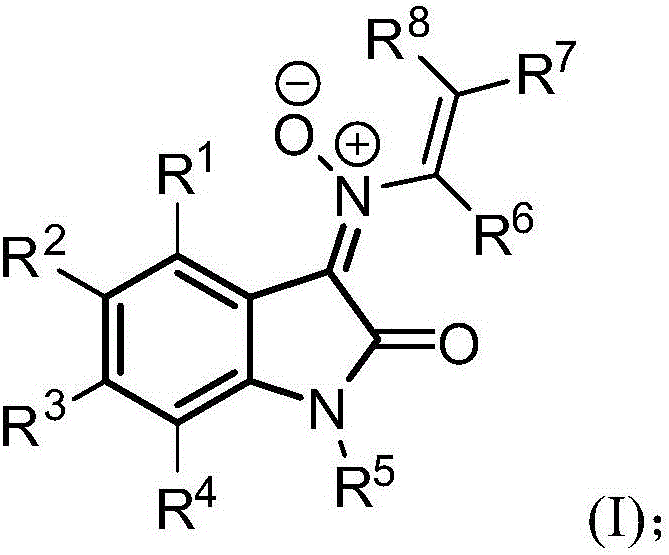
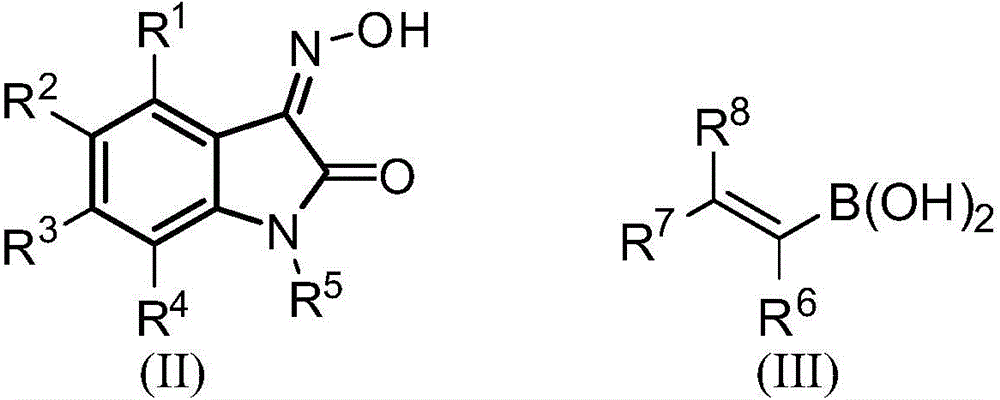
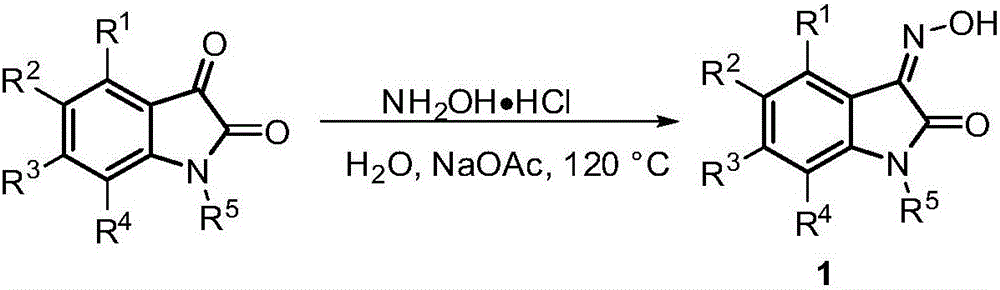
2,3-indolinone-3-N-alkenylnitrone cycloaddition derivative and synthesis method and application thereof
A synthetic method, the technology of indoledione, which is applied in the field of medicine, can solve the problems of difficult synthesis of raw materials, narrow application range of substrates, and low yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Synthesize the 2,3-indoledione-3-N-enylnitrone derivatives of the present invention according to the following synthetic route.
[0077]
[0078] Put 2,3-indoledione-3-oxime substrate 1 (0.3mmol) and alkenyl boronic acid 2 (0.9mmol) in a reaction tube, add 3mL of methanol, stir the reaction at 25°C for 5-20h, spin Remove the solvent, add water (10mL) to the resulting reactant, extract with dichloromethane (2×10mL), combine the organic phases, dry with anhydrous sodium sulfate, filter, remove the solvent under reduced pressure, and separate by silica gel column chromatography (petroleum ether / Ethyl acetate=10:1~1:1, volume ratio), the target product 3 was obtained. The different target products and their characterization are as follows:
[0079] 3aa: Orange-red solid, 68mg, 93% yield; mp 170-171℃. 1 H NMR (400MHz, DMSO–d 6 ):δ10.94(s,1H),8.78(dt,J=13.2,1.4Hz,1H),8.22(d,J=7.6Hz,1H),7.34(t,J=7.7Hz,1H),7.02 (t,J=7.7Hz,1H),6.95-6.85(m,2H),2.27(q,J=7.3Hz,2H),1.49-1.41...
Embodiment 2
[0116] Synthesize the 2,3-indoledione-3-N-enylnitrone derivatives of the present invention according to the following synthetic route.
[0117]
[0118] Put 2,3-indoledione-3-oxime substrate 1 (0.3mmol), alkenyl boronic acid 2 (0.9mmol), cuprous bromide (0.3mmol), triethylamine (3mmol) in the reaction tube , add 3mL of 1,2-dichloroethane, stir the reaction at 60°C for 5-20h, remove the solvent from the reactant under reduced pressure, and separate by silica gel column chromatography (dichloromethane / methanol=100:1~10:1, volume ratio), the target product 3 was obtained. The different target products and their characterization are as follows:
[0119] 3al: Orange-red solid, 52mg, 76% yield; mp 144-145℃. 1 H NMR (500MHz, DMSO–d 6 ): δ10.94(s, 1H), 8.37(d, J=7.6Hz, 1H), 7.55(t, J=7.7Hz, 1H), 7.21(t, J=7.6Hz, 1H), 7.06(d ,J=7.8Hz,1H),6.31(d,J=7.7Hz,1H),2.89(d,J=5.9Hz,2H),2.69(m,2H),2.27-2.19(m,2H); 13 C NMR (125MHz, DMSO–d 6 ): δ160.6, 146.9, 141.2, 135.0, 132.8, 128.6, 12...
experiment example 1
[0133] Experimental Example 1: The 2,3-indoledione-3-N-enylnitrone derivatives of the present invention were tested for their inhibitory activity against various human tumor strains in vitro:
[0134] (1) Cell culture: MGC-803, HepG2, NCI-H460, 7702 cells were cultured in DMEM containing 10% (volume ratio) fetal bovine serum and 1% (volume ratio) double antibody (containing penicillin and streptomycin) Medium, at a temperature of 37°C, 5% CO 2 and 95% air incubator, and change the medium every other day. After the cells were confluent, they were passaged and frozen.
[0135] (2) Seed plate: Take cells in the logarithmic growth phase, remove the old medium, wash twice with PBS, digest the cells with trypsin, add new medium after the cells become round, stop the digestion of the cells and pipette the suspended cells to prepare into a single cell suspension. Take an appropriate amount of cell suspension, add a certain amount of medium to dilute, inoculate into a 96-well plate,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com