Catalyst for preparing dimethyl carbonate and preparation method of catalyst
A technology of dimethyl carbonate and catalyst, which is applied in the field of research and development of new catalysts and the field of dimethyl carbonate production, which can solve the problems of low catalyst activity and achieve the effects of improving stability, increasing activity and inhibiting agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
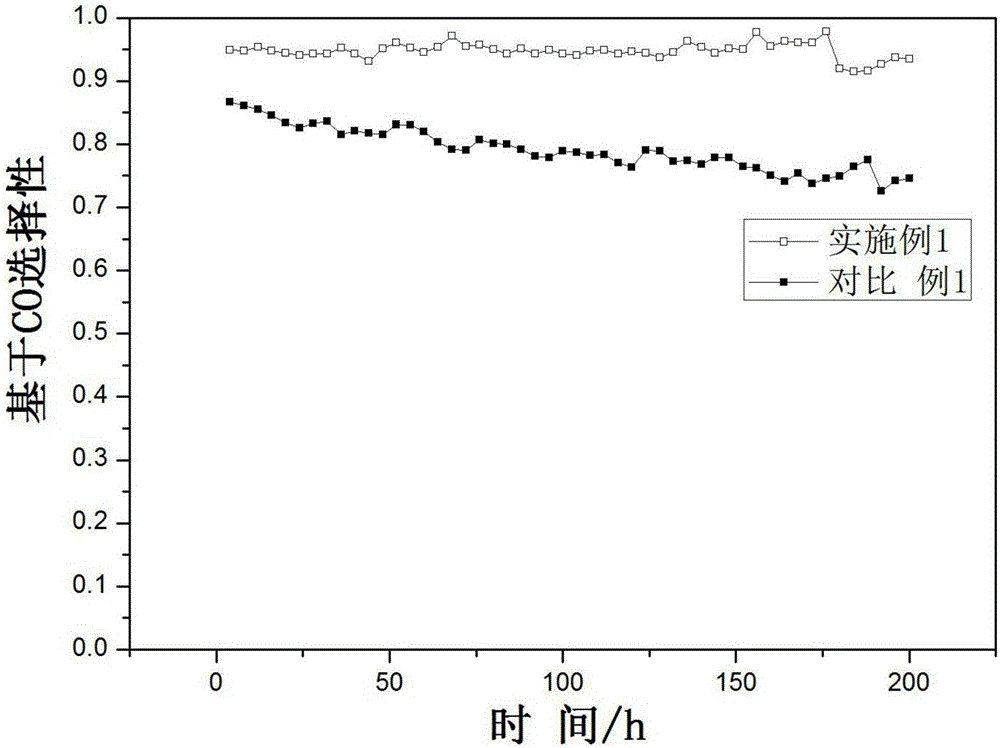
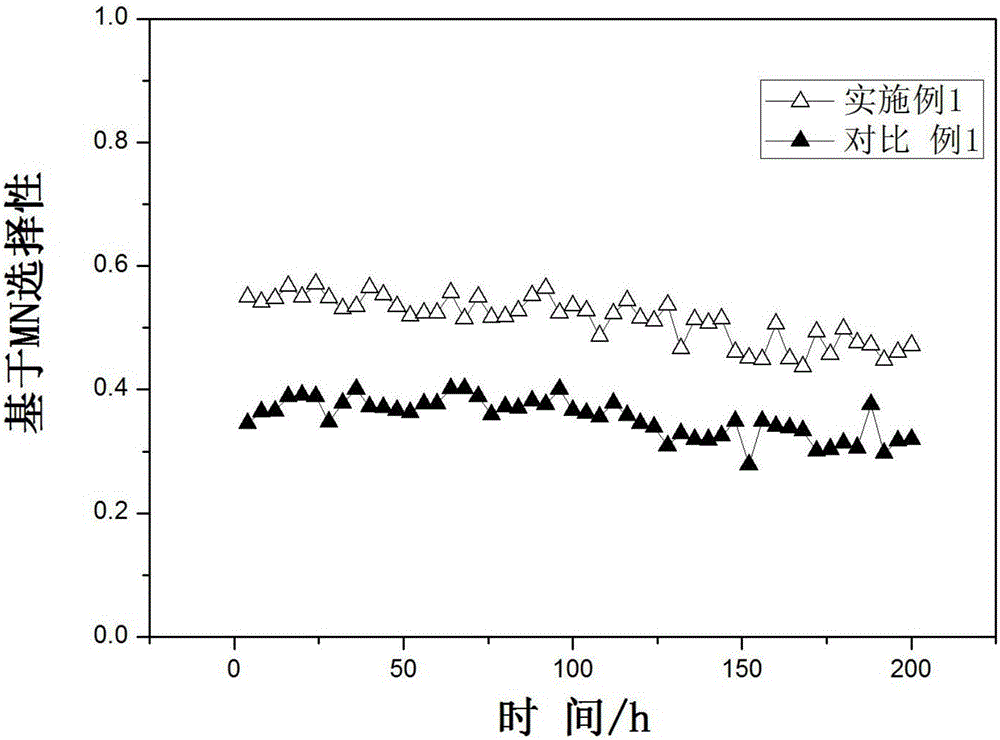
Image
Examples
Embodiment 1
[0023] A. Add 0.25g Pd(NO 3 ) 2 2H 2 O and 0.38g Cu(NO 3 ) 2 ·3H 2 O, after dissolving in water respectively, mix and dilute with water to 100ml, use ammonia water and dilute nitric acid to adjust the pH to 10. Slowly pour the solution into a round bottom flask containing 10g of alkali-treated NaY molecular sieve, 30℃ magnetic force Stir for 4 hours to fully exchange the metal cations in the main active component precursor and co-active component precursor with the cations in the carrier.
[0024] B. Then weigh 1g of PVP and add it to the mixture, raise the temperature to 60°C, and continue to stir for 4h. Suction filtration, fully washed with 500ml of distilled water, and dried at 120°C for 6 hours to obtain the catalyst.
Embodiment 2
[0026] A. Add 0.25g Pd(NO 3 ) 2 2H 2 O, 0.38g Cu(NO 3 ) 2 ·3H 2 O and 0.5 g KNO 3 , respectively dissolved in water, mixed and diluted with water to 100ml, using ammonia and dilute nitric acid to adjust the pH to 10. Slowly pour the solution into a round-bottomed flask containing 10g of alkali-treated NaY molecular sieve, and stir magnetically at 30°C 4h, the metal cations in the main active component precursor and the co-active component precursor are fully exchanged with the cations in the carrier.
[0027] B. Then weigh 2g of PVP and add it to the mixture, raise the temperature to 60°C, and continue to stir for 4h. Suction filtration, fully washed with 500ml of distilled water, and dried at 200°C for 6 hours to obtain the catalyst.
Embodiment 3
[0029] A. Add 0.25g Pd(NO 3 ) 2 2H 2 O and 0.57g Cu(NO 3 ) 2 ·3H 2 O, and 1g KNO 3 , respectively dissolved in water, mixed and diluted with water to 100ml, adjusted to pH 10 with ammonia water and dilute nitric acid.
[0030] B. Weigh 1g of PVP and add it into the mixture, stir at room temperature for 2h until the organic ligands are completely dissolved. Slowly pour the solution into a round-bottomed flask equipped with 10 g of alkali-treated NaY molecular sieves, stir magnetically at 30°C for 5 hours, and make the metal cations in the main active component precursor and the auxiliary active component precursor and the carrier The cations were fully exchanged, the temperature was raised to 70°C, and stirring was continued for 5h. Suction filtration, fully washed with 1000ml of distilled water, and dried at 160°C for 6 hours to obtain the catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com