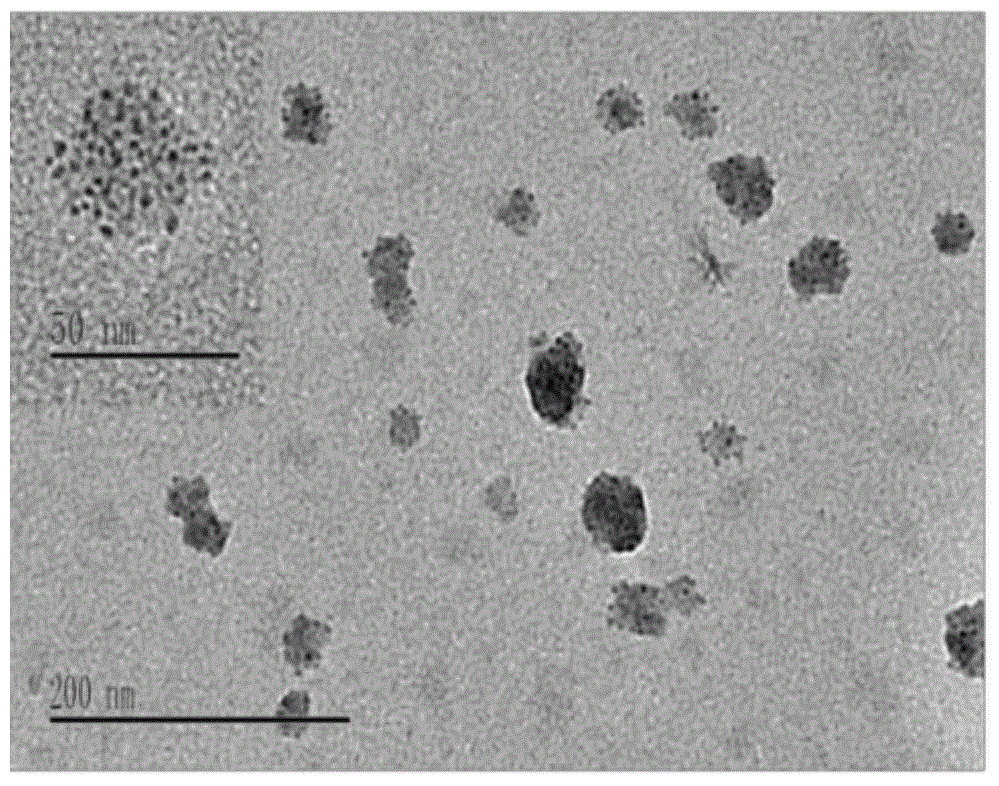
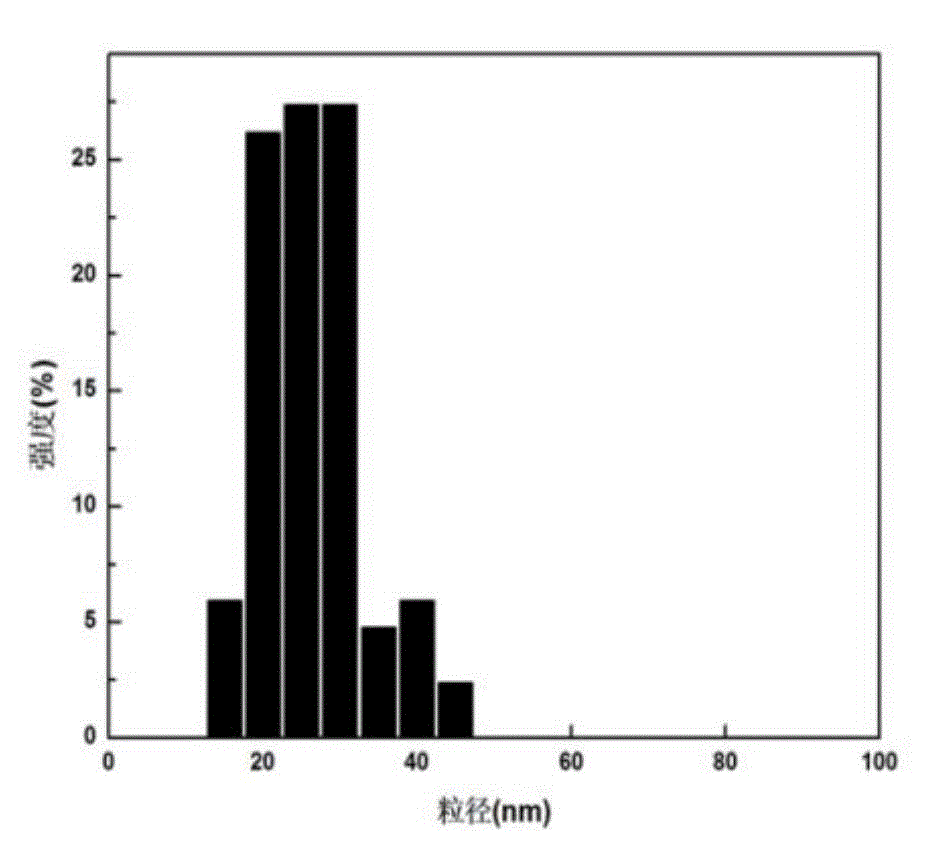
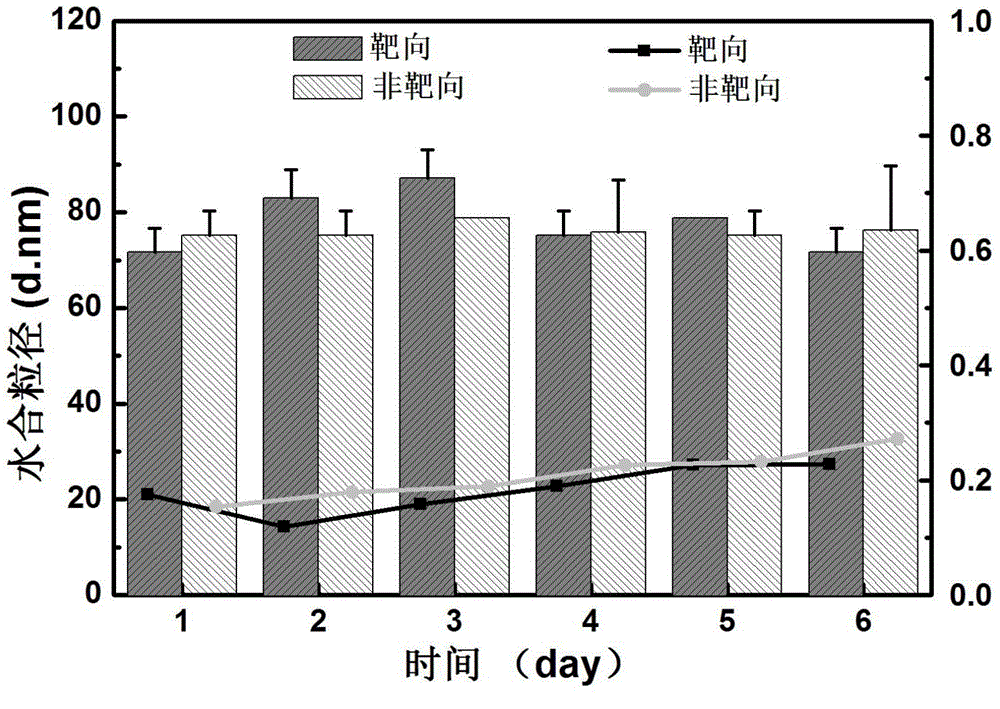
Tumor-targeted nuclear magnetic resonance/fluorescence dual-mode imaging contrast agent, preparation and applications thereof
A dual-mode imaging and nuclear magnetic resonance technology, which is applied in preparations for in vivo experiments, emulsion delivery, drug delivery, etc., can solve the problems of lack of tumor targeting and easy aggregation of probes, and achieve enhanced MRI signal intensity and near-infrared fluorescence signal intensity, high quantum yield, and low toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of tumor-targeted nuclear magnetic resonance and near-infrared fluorescence dual-mode imaging contrast agent:
[0058] (1) Preparation of NMR amphiphile: Take 1.0026g of tri-tert-butyl 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and dissolve it in dichloromethane (DCM), and 0.2448g N-hydroxysuccinimide (NHS) under the action of 1.8087g dicyclohexylcarbodiimide (DCC), activated at room temperature for 2h, then added 0.5065g hexadecylamine, stirred at room temperature for 12h , after suction filtration, the reaction product was precipitated with a large amount of ether, and vacuum-dried to obtain a milky white powder; then added to a mixture of trifluoroacetic acid (TFA) and DCM, stirred and reacted for 2 hours, the reaction solvent was removed by rotary evaporation, and the reaction product was washed with petroleum ether. Vacuum-dried to obtain a white oil; redissolve the white oil in water, add 0.3481 g of gadolinium acetate hydrate at t...
Embodiment 2
[0063] Preparation of tumor-targeted nuclear magnetic resonance and near-infrared fluorescence dual-mode imaging contrast agent:
[0064] (1) Preparation of NMR amphiphile: Take 1.0026g of tri-tert-butyl 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and dissolve it in dichloromethane (DCM), and 0.2448g N-hydroxysuccinimide (NHS) under the action of 1.8087g dicyclohexylcarbodiimide (DCC), activate at room temperature for 2h, then add 0.5660g carbooctadecylamine, stir at room temperature Reaction for 12 hours, after suction filtration, precipitate the reaction product with a large amount of ether, and dry it in vacuum; then add it to the mixture of trifluoroacetic acid (TFA) and DCM, stir and react for 2 hours, remove the reaction solvent by rotary evaporation, wash the reaction product with petroleum ether, vacuum Dry to obtain a white oil; redissolve the white oil in water, add gadolinium acetate hydrate at the same time, adjust the pH of the solution to 5-6,...
Embodiment 3
[0069] Preparation of tumor-targeted nuclear magnetic resonance and near-infrared fluorescence dual-mode imaging contrast agent:
[0070](1) Preparation of NMR amphiphile: Take 1.0026g of tri-tert-butyl 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and dissolve it in dichloromethane (DCM), and 0.2448g N-hydroxysuccinimide (NHS) under the effect of 1.8087g dicyclohexylcarbodiimide (DCC) activated at room temperature for 2h, then added 1.571g phospholipids, stirred at room temperature for 12h, pumped After filtration, the reaction product was precipitated with a large amount of diethyl ether, dried in vacuo; then added to a mixture of trifluoroacetic acid (TFA) and DCM, stirred for 2 hours, and the reaction solvent was removed by rotary evaporation, the reaction product was washed with petroleum ether, and dried in vacuo to obtain a white oil matter; redissolve the white oil in water, add gadolinium acetate hydrate at the same time, adjust the pH of the solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Relaxation rate | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com