Method for recycling magnesium from heavy metal sludge to prepare magnesium hydroxide fire retardant
A technology of heavy metal sludge and magnesium hydroxide, applied in the direction of magnesium hydroxide, process efficiency improvement, etc., can solve the problems of environmental burden and resource waste, unreasonable utilization of magnesium resources, low purity of cobalt, nickel and manganese, etc., and achieves the recovery process. Reasonable and easy to implement, considerable economic value, and the effect of high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
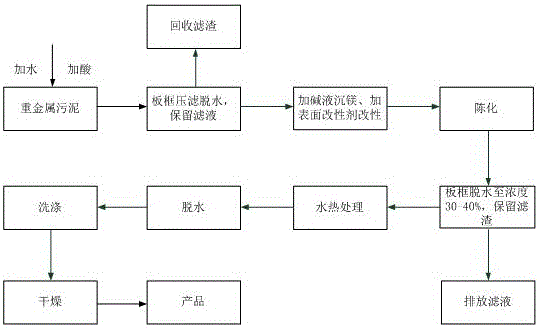
[0027] A method for reclaiming magnesium from heavy metal sludge, comprising the following steps:
[0028] Pretreatment water washing: add heavy metal sludge to pure water according to the mass ratio of pure water to sludge dry matter 8:1, turn on the electric furnace to heat up, and adjust the pH to 7.0 with 0.4g / L dilute sulfuric acid aqueous solution while stirring (the temperature is finally stabilized at 80°C, pH=7.0 is measured at this temperature), continue to stir for 10 minutes, the obtained water residue mixture is dehydrated by plate and frame pressure filtration, and filtrate 1 and filter residue 1 are obtained; filter residue 1 is washed with hot water at 80-90°C, filtered and dehydrated 1 time, the filtrate 2 was obtained. The filtrate obtained by combining filtrate 1 and filtrate 2 is magnesium liquid. Magnesium in the magnesium solution accounts for more than 99% of the magnesium in the heavy metal sludge, the content of Ni is 0.5mg / L, Co, Mn, and Zn are not d...
Embodiment 2
[0030] A kind of method based on embodiment 1 reclaims magnesium from heavy metal sludge and prepares magnesium hydroxide flame retardant, comprises the following steps:
[0031] 1. Pretreatment water washing: add heavy metal sludge to pure water according to the mass ratio of pure water to sludge dry matter 8:1, turn on the electric furnace to heat up, and adjust the pH to 7.0 with 0.4g / L dilute sulfuric acid aqueous solution while stirring (the temperature is at the end Stable at 80°C, pH=7.0 is measured at this temperature), continue to stir for 10 minutes, the obtained water residue mixture is dehydrated by plate and frame pressure filtration, and filtrate 1 and filter residue 1 are obtained; filter residue 1 is washed with hot water at 80-90°C Filter and dehydrate once to obtain filtrate 2. The filtrate obtained by combining filtrate 1 and filtrate 2 is magnesium liquid. Magnesium in the magnesium solution accounts for more than 99% of the magnesium in the heavy metal sl...
Embodiment 3
[0040] A method for reclaiming magnesium from heavy metal sludge, comprising the following steps:
[0041] Pretreatment water washing: add heavy metal sludge to pure water according to the mass ratio of pure water to sludge dry matter 15:1, turn on the electric furnace to heat up, and adjust the pH to 8.0 with 0.6g / L dilute sulfuric acid aqueous solution while stirring (the temperature is finally stabilized at 85°C, pH=8.0 is measured at this temperature), continue to stir for 15 minutes, and dehydrate the water residue mixture through plate and frame pressure filtration to obtain filtrate 1 and filter residue 1; filter residue 1 is washed with hot water at 80-90°C, filtered and dehydrated 3 times to obtain the filtrate 2. The filtrate obtained by combining filtrate 1 and filtrate 2 is magnesium liquid. Magnesium in the magnesium solution accounts for more than 99% of the magnesium in the heavy metal sludge, the content of Ni is 0.6mg / L, Co, Mn, and Zn are not detected, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com