Method for synthesizing quinoline derivative
A synthesis method and a technology of derivatives, applied in the field of quinoline derivatives, can solve the problems of limited catalyst activity, high reaction temperature, and difficult to handle by-products, and achieve the omission of protection and deprotection synthesis steps, simple reaction substrates, and easy reaction The effect of cheap substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
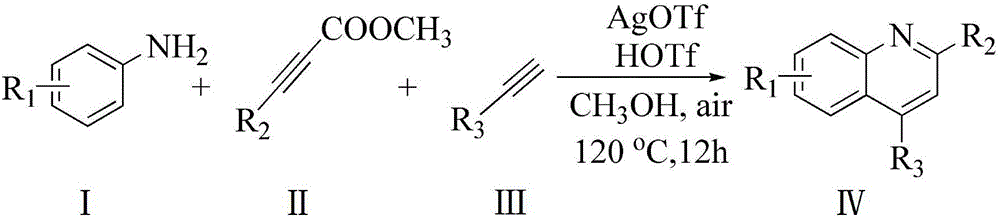
[0026] A kind of synthetic method of 4-phenylquinoline-2-formic acid methyl ester, comprises the following steps:
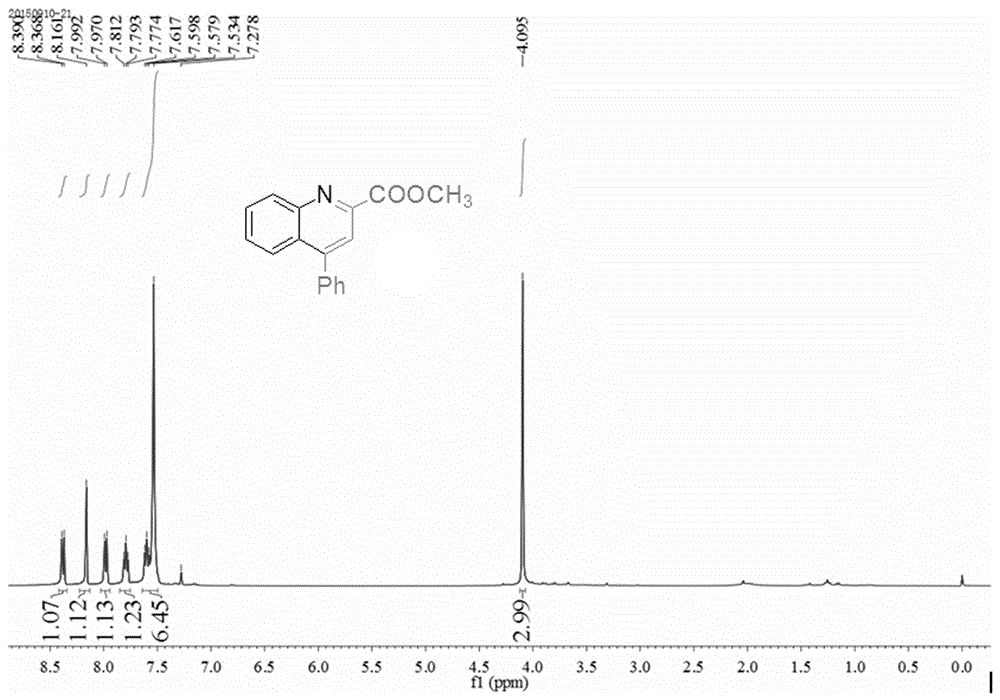
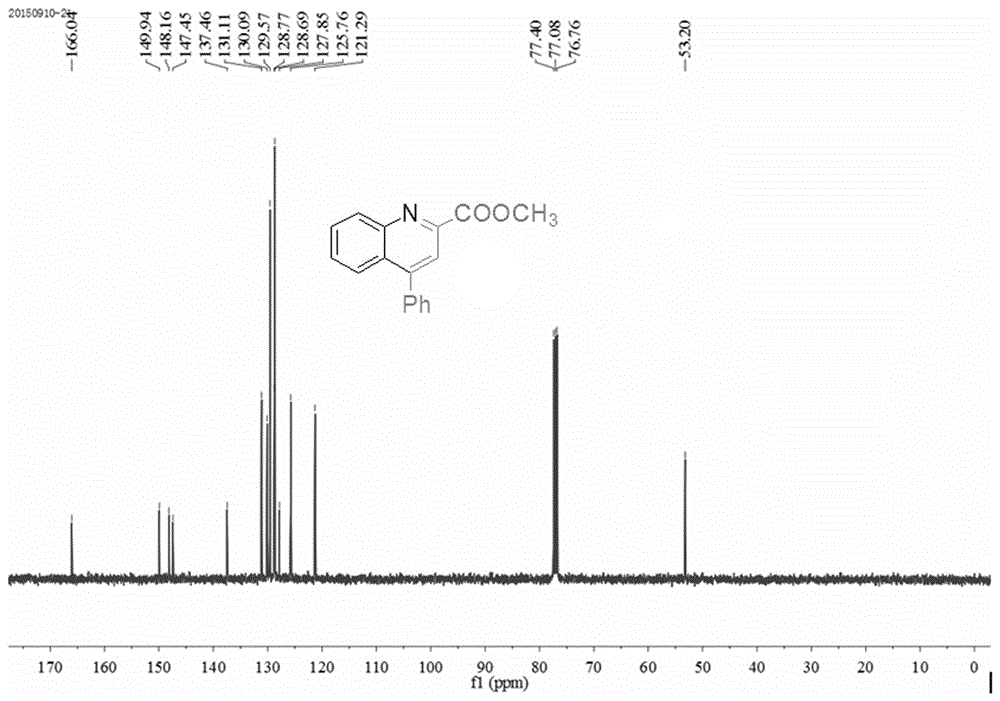
[0027] 1.0mmol (93mg) of aniline, 1.0mmol (142.1mg) of dimethyl butynedate, 1.2mmol (122.4mg) of phenylacetylene, 0.005mmol (1.3mg) of catalyst AgOTf, and 0.01mmol (1.5 mg), solvent hexanitrile 2mL, reacted in an oil bath at 100°C for 8h, cooled to room temperature, added 5mL of water, extracted three times with ethyl acetate, combined the organic layers, concentrated under reduced pressure, and the product was purified by column chromatography, 300-400 mesh Silica gel column, the eluent was a mixture of ethyl acetate and petroleum ether, the volume ratio of the two was 1:10, and 235.1 mg of a white solid product was obtained with a yield of 89% and a purity of 99.9%. 1H NMR (400MHz, CDCl 3 )δppm: 8.38(d, J=8.8Hz, 1H), 8.16(s, 1H), 7.98(d, J=8.8Hz, 1H), 7.77-7.81(t, 1H), 7.53-7.62(m, 6H ),4.10(s,3H); 13 C NMR (100MHz, CDCl 3 )δppm: 166.0, 149.9, 148.2, 147.5,...
Embodiment 2
[0031] A synthetic method of 6-fluoro-4-phenylquinoline-2-carboxylic acid methyl ester, comprising the following steps:
[0032] Add 1.0mmol (111mg) of p-fluoroaniline, 1.0mmol (142.1mg) of dimethyl butyndioate, 1.2mmol (122.4mg) of phenylacetylene, 0.005mmol (1.3mg) of catalyst AgOTf, and 0.01mmol of HOTf in the reaction vessel (1.5mg), solvent nitrile 2mL, reacted in 120°C oil bath for 12h, cooled to room temperature, added 5mL of water, extracted three times with ethyl acetate, combined organic layers, concentrated under reduced pressure, the product was purified by column chromatography, 300- 400-mesh silica gel column, the eluent was a mixture of ethyl acetate and petroleum ether, the volume ratio of the two was 1:10, and 220.0 mg of a white solid product was obtained with a yield of 78% and a purity of 99.9%. 1 H NMR (400MHz, CDCl 3 )δppm: 7.38 (d, J=8.8Hz, 1H), 8.17 (s, 1H), 7.52-7.54 (m, 7H), 4.09 (s, 3H); 13 C NMR (100MHz, CDCl 3 )δppm: 165.8, 163.2, 160.7, 149.4 (...
Embodiment 3
[0036] A kind of synthetic method of 6-chloro-4-phenylquinoline-2-formic acid methyl ester, comprises the following steps:
[0037] Add 1.0mmol (127mg) of p-chloroaniline, 1.0mmol (142.1mg) of dimethyl butyndioate, 1.2mmol (122.4mg) of phenylacetylene, 0.005mmol (1.3mg) of catalyst AgOTf, and 0.01mmol of HOTf in the reaction vessel (1.5mg), solvent nitrile 2mL, reacted in an oil bath at 100°C for 12h, cooled to room temperature, added 5mL of water, extracted three times with ethyl acetate, combined organic layers, concentrated under reduced pressure, the product was purified by column chromatography, 300- 400-mesh silica gel column, the eluent was a mixture of ethyl acetate and petroleum ether, the volume ratio of the two was 1:5, and 241.4 mg of a white solid product was obtained with a yield of 81% and a purity of 99.9%. 1 HNMR (400MHz, CDCl 3 )δppm: 8.31(d, J=9.2Hz, 1H), 8.17(s, 1H), 7.95(s, 1H), 7.74(d, J=8.8Hz, 1H), 7.51-7.60(m, 5H), 4.10(s,3H); 13 C NMR (100MHz, CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com