Paclitaxel dimer, preparation method and preparation thereof
A paclitaxel dimer technology, applied in the field of paclitaxel dimer preparation, can solve the problem of low solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention also provides a preparation method of paclitaxel dimer, the method comprising:
[0033] Step 1: Mixing the paclitaxel solution and linker molecules to obtain a mixed solution;
[0034] Step 2: adding EDC and DMAP to the mixed solution obtained in Step 1 to react to obtain paclitaxel dimers;
[0035] According to the present invention, paclitaxel is first dissolved in an organic solvent to obtain a paclitaxel solution. The organic solvent is not particularly limited as long as it can dissolve paclitaxel, preferably dichloromethane, chloroform, dimethyl sulfoxide or dimethylformamide; the mass concentration of the paclitaxel solution is preferably 10-20mg mL -1 .
[0036] According to the present invention, the above-mentioned paclitaxel solution and linker molecules are mixed and fully dissolved to obtain a mixed solution; the molar ratio of the paclitaxel and linker molecules is preferably 1:(0.55-0.56).
[0037] According to the present inventi...
Embodiment 1
[0052] Example 1: Synthesis of Paclitaxel Dimer Coupled by Suberic Acid
[0053] Weigh paclitaxel (molecular weight is 853.92) (136mg, 0.16mmol) and dissolve in dichloromethane, then add suberic acid (15.5mg, 0.089mmol), EDC (67.1mg, 0.35mmol) and DMAP (2.2mg, 0.018mmol) , After 1 hour, EDC (32.6mg, 0.17mmol) and DMAP (2.2mg, 0.018mmol) were added again, and after reacting at room temperature for 24 hours, filter with an oil phase filter head, then use dichloro and ethyl acetate from 8 :1 to 2:1 slowly increase the polarity to wash the column until the product flows down, and finally spin dry the dichloride and ethyl acetate in the product to obtain a solid powder.
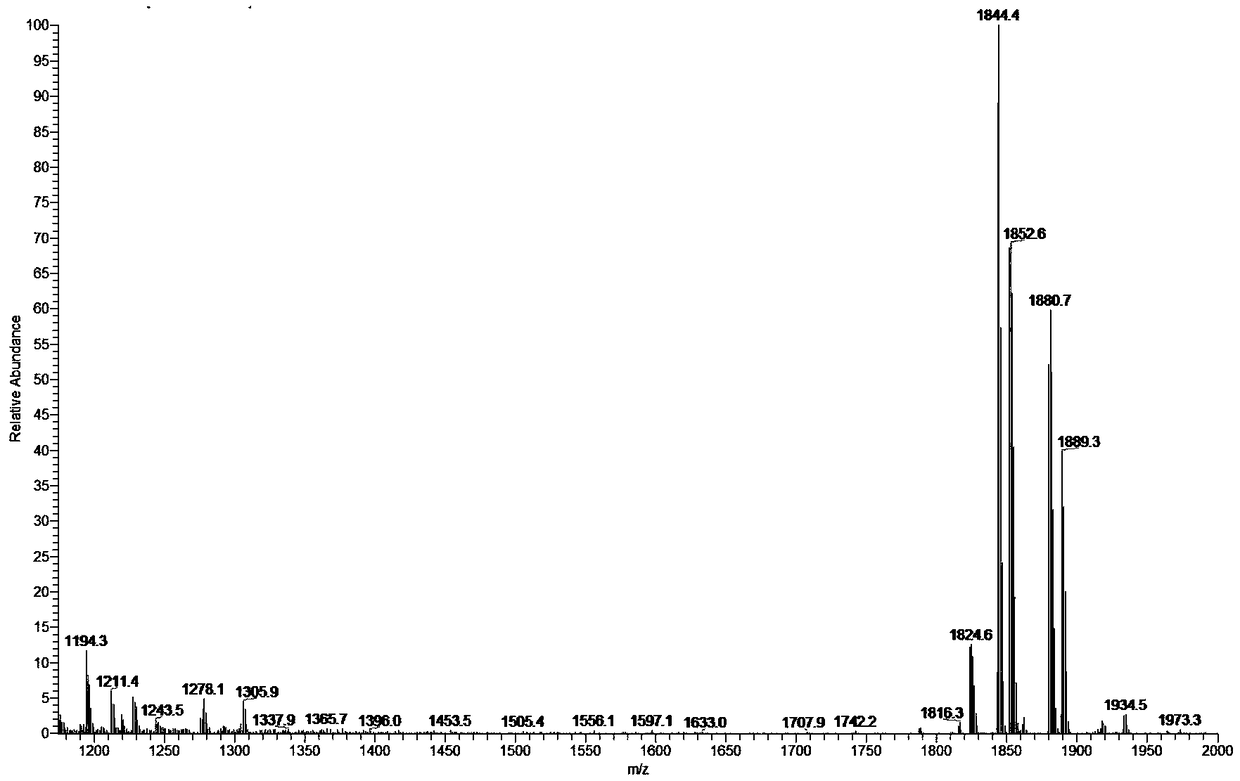
[0054] figure 1 The mass spectrum of paclitaxel dimer prepared for Example 1 of the present invention, figure 1 It shows that the present invention has successfully prepared suberic acid-coupled paclitaxel dimer.
Embodiment 2
[0055] Embodiment 2: Synthesis of paclitaxel dimer containing disulfide bond
[0056] Weigh paclitaxel (molecular weight is 853.92) (204.9mg, 0.24mmol) and dissolve in dichloromethane, then add 3,3'-dihydrooxycholic acid (27.3mg, 0.13mmol), EDC (97.77mg, 0.51mmol) and DMAP (3.05mg, 0.025mmol), after 1 hour, EDC (47.93mg, 0.25mmol) and DMAP (3.05mg, 0.025mmol) were added again, reacted at room temperature for 48 hours, filtered with an oil phase filter, and then washed with two Chlorine and ethyl acetate slowly increase the polarity from 8:1 to 2:1 to wash the column until the product flows down, and finally the organic solvent in the product is spin-dried to obtain a solid powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com