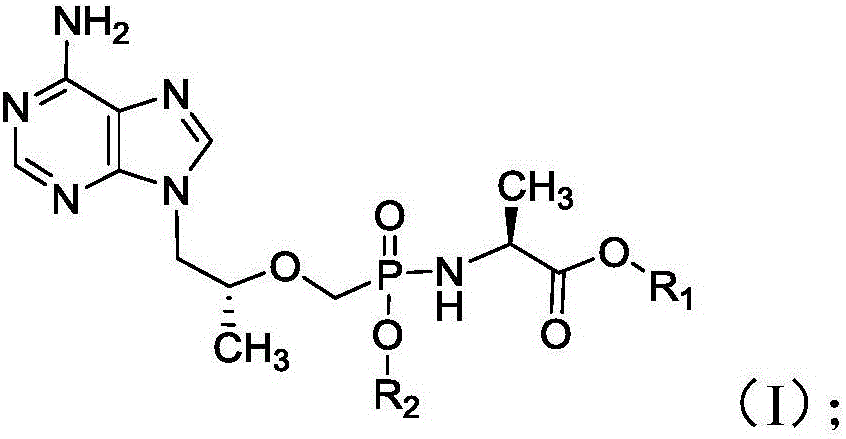
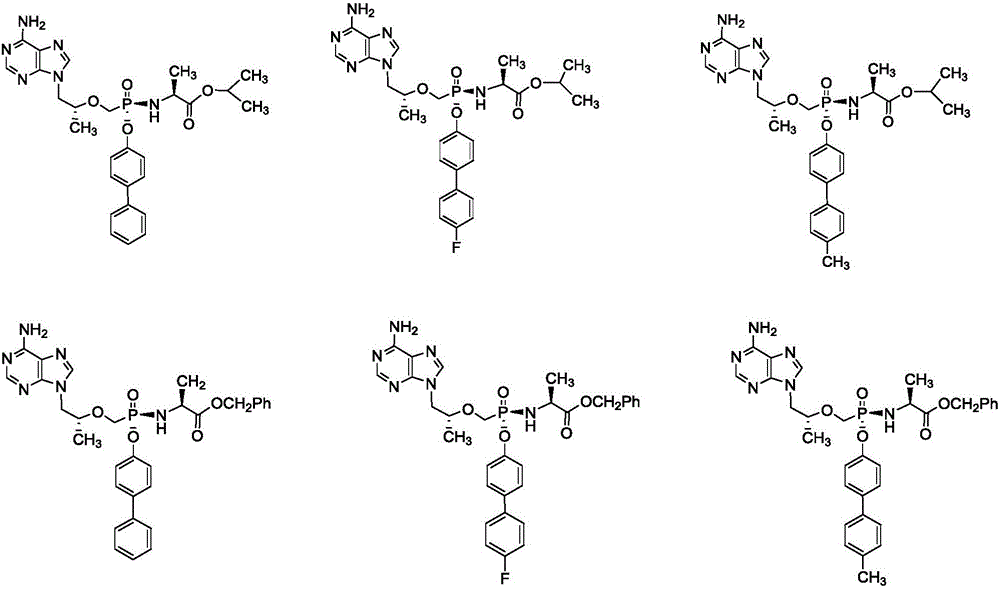
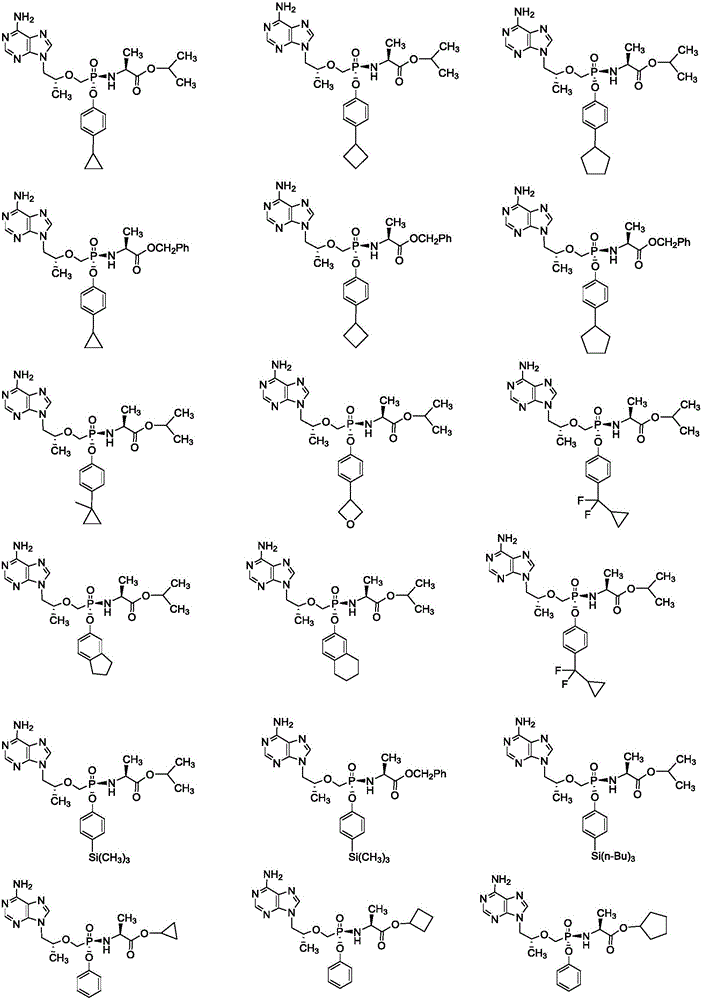
Phosphamide nucleosides compound, pharmaceutically acceptable salt and application thereof, and pharmaceutical composition
A technology for phosphoramide nucleosides and compounds is applied in pharmaceutical compositions, phosphoramide nucleosides and pharmaceutically acceptable salts and application fields thereof, and can solve problems such as nephrotoxicity and instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] 1-(((cyclohexyloxy)carbonyl)oxy)ethyl(((((R)-1-(6-amino-9H-purin-9-yl)propan-2-yl)oxy)methyl base) (phenoxy)phosphoryl)-L-alanine ethyl ester fumarate (6)
[0089]
[0090] Step 1: Synthesis of 1-(((cyclohexyloxy)carbonyl)oxy)ethyl(tert-butoxycarbonyl)-L-alanine (3)
[0091]
[0092] Under the protection of nitrogen, the DMF (15ml) solution of Boc-L-alanine (2.0g, 10.5mmol) was added dropwise to the DMF (16mL) reaction solution of potassium carbonate (2.9g, 21.1mmol) at 0°C, dropwise After completion, react at room temperature for 30 minutes, cool to 0°C, compound 2 (4.3 g, 21.1 mmol) is added dropwise to the reaction solution, and rise to room temperature to react overnight. The reaction solution was poured into ice water, extracted with ethyl acetate, the organic phase was dried over anhydrous sodium sulfate, concentrated, and the residue was subjected to column chromatography with petroleum ether:ethyl acetate=20:1 to obtain compound 3, 2.0g, 53% .
[0093] ...
Embodiment 2
[0110] Example 2 ((isopropyl)oxy)methyl ((((R)-1-(6-amino-9H-purin-9-yl)prop-2-yl)oxy)methyl)( Phenoxy)phosphoryl)-L-alanine (10)
[0111]
[0112] Step 1: Synthesis of ((isopropoxycarbonyl)oxy(tert-butoxycarbonyl)-L-alanine (8)
[0113]
[0114]A solution of N-Boc-L-alanine (2.5g, 13.2mmol) in DMF (10ml) was added dropwise to ice-bathed potassium carbonate (3.65g, 26.4mmol) and sodium iodide (3.96g, 26.4mmol) in DMF (30 mL) mixture was stirred at room temperature for 30 minutes, cooled to 0°C, compound 7 (4.03 g, 26.4 mmol) was added, and stirred overnight at room temperature. The reaction solution was poured into water, extracted with ethyl acetate, and the obtained organic phase was anhydrous Na 2 SO 4 Drying, silica gel column chromatography (eluent: petroleum ether: ethyl acetate = 20:1) gave compound 8, 2.46g, 61%. 1 H NMR (400MHz, CDCl 3 ): δ1.31-1.44 (m, 18H), 4.35-4.39 (m, 1H), 4.91-4.94 (m, 1H), 5.03-5.04 (m, 1H), 5.74-5.84 (m, 2H).
[0115] Step 2: Synth...
Embodiment 3
[0123] Example 3: ((((((R)-1-(6-amino-9H-purin-9-yl)prop-2-yl)oxy)methyl)(phenoxy)phosphoryl)- L-alanyl)oxy)methyl pivalate (14)
[0124]
[0125] Step 1: Synthesis of (((tert-butoxycarbonyl)-L-alanyl)oxy)methylpivalate (12)
[0126]
[0127] Boc-L-alanine (2g, 10.5mmol) in DMF (9mL) was added dropwise to ice-bathed potassium carbonate (2.92g, 21.1mmol) and sodium iodide (3.16g, 21.1mmol) in DMF (28mL) The mixture was stirred and reacted at room temperature for 30 minutes, then cooled to 0°C, compound 11 (3.18 g, 21.1 mmol) was added, and stirred overnight at room temperature. The reaction solution was poured into water, extracted with ethyl acetate, and the obtained organic phase was anhydrous Na 2 SO 4 Drying, silica gel column chromatography (eluent: petroleum ether: ethyl acetate = 20:1) gave compound 12, 1.49g, 47%. 1 H NMR (400MHz, CDCl 3 ): δ1.18-1.58 (m, 21H), 4.22-4.31 (m, 1H), 4.99 (m, 1H), 5.72-5.84 (m, 2H).
[0128] Step 2: Synthesis of ((L-alanyl)oxy)m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com