Tripterine nanosuspension and preparation method thereof
A technology of nano-suspension and tripterine, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the limitations of drug delivery methods, low bioavailability, and toxicity. Problems such as large side effects, to achieve the effects of economical, safe and easy-to-obtain excipients, high tumor cell inhibition rate, and simple prescription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
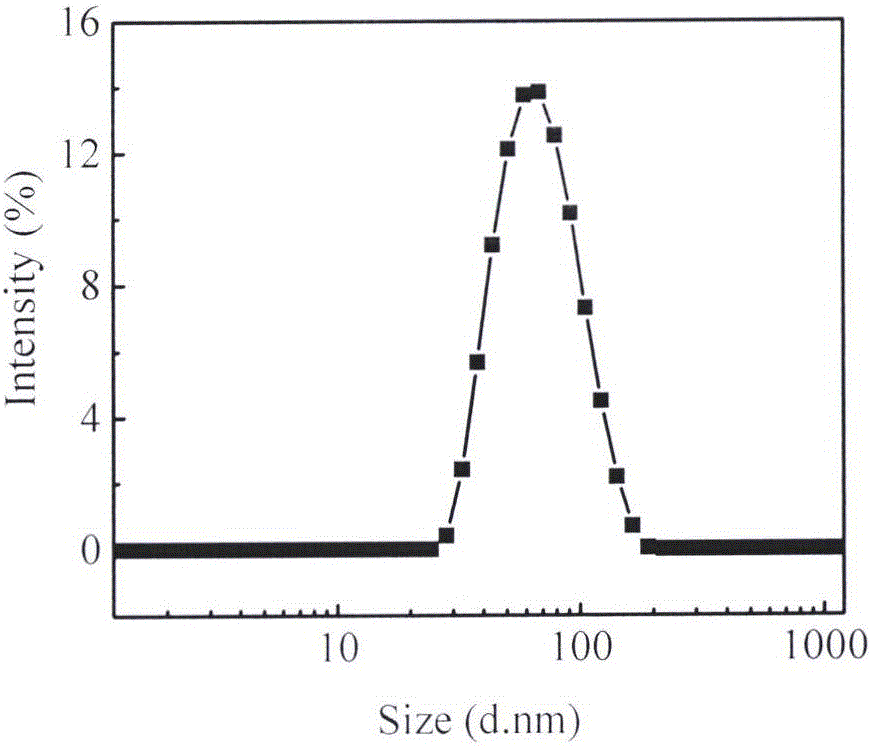
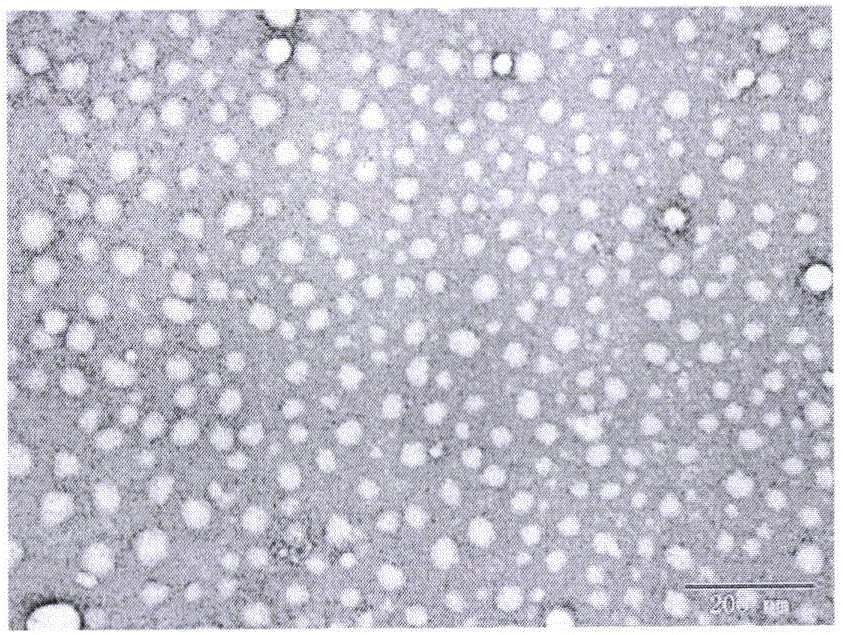
[0031] Dissolve 5mg tripterine (CSL) and 5mg PEG2000-PCL2000 in 0.2mL methanol, slowly instill in 5mL deionized water at room temperature at 250HZ, continue to sonicate for 3min, remove methanol by rotary evaporation, and obtain CSL nanosuspension agent. The average particle size is 67.1nm ( figure 1 ), the polydispersity index (PDI) was 0.232, and the potential value was -10.4mV.
Embodiment 2
[0033] Dissolve 5mg of tripterine (CSL) and 5mg of PEG2000-PCL1140 in 0.2mL of methanol, slowly instill in 5mL of deionized water with 250HZ ultrasound at room temperature, continue to sonicate for 3min, remove methanol by rotary evaporation, and obtain CSL nanosuspension agent. The average particle diameter is 74.2nm, the polydispersity index (PDI) is 0.201, and the potential value is -12.6mV.
Embodiment 3
[0035] Dissolve 5mg tripterine (CSL) and 5mg PEG5000-PCL2000 in 0.2mL methanol, slowly instill in 5mL deionized water with 250HZ ultrasound at room temperature, continue to sonicate for 3min, remove methanol by rotary evaporation, and obtain CSL nanosuspension agent. The average particle size is 76.4nm, the polydispersity index (PDI) is 0.192, and the potential value is -13.8mV.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com