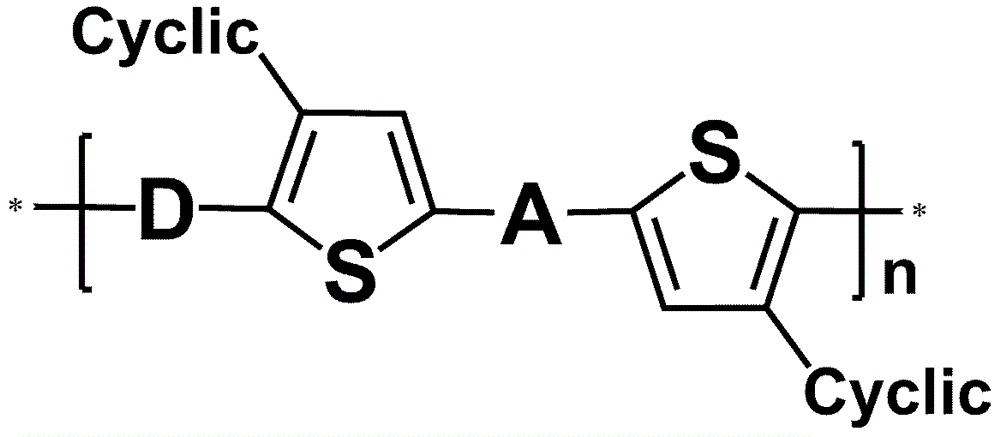
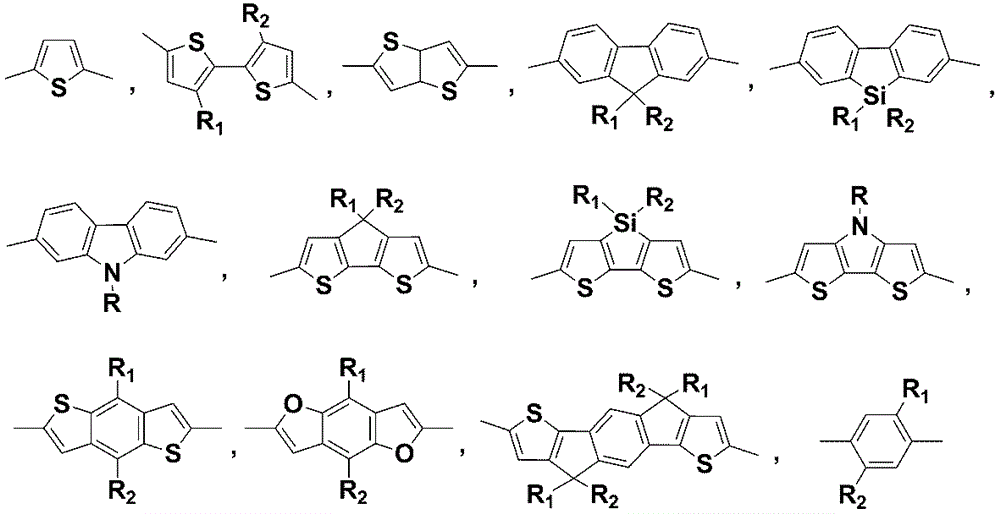
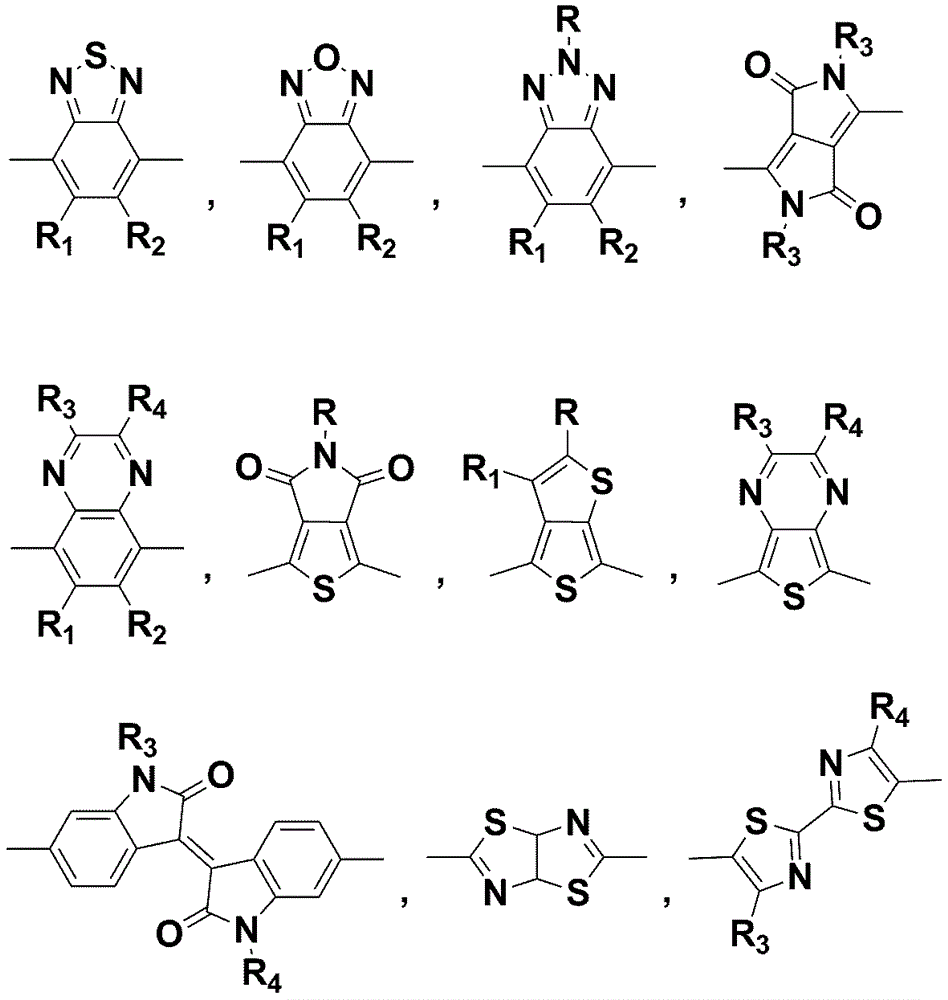
Application of annular alkyl chain substituted semiconductor polymer in organic solar cell
A technology of alkyl chains and semiconductors, applied in the field of donor-acceptor narrow-bandgap semiconducting polymers, which can solve the problems of great influence on stacking characteristics, disordered arrangement, low symmetry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Based on cyclopentyl substitution of 4,7-dithiophene benzo[ c ][1,2,5]Thiadiazole (DTBT) and BDT Alternating Copolymer Preparation, the detailed route is attached Figure 4 .
[0033] (1) 3-cyclopentylthiophene (TH- c 5) Synthesis of:
[0034]Add 1.2 g (50 mmol) magnesium and 1 grain of iodine into a 100 mL three-necked flask, add 30 mL anhydrous ether through a syringe under argon protection, slowly add 7.45 g (50 mmol) bromocyclopentane dropwise, and react to After the magnesium disappeared, the reaction bottle was placed in an ice bath, and 30 mg Ni(dppp)Cl was added 2 , and dropwise added 6.52 g (40 mmol) tribromothiophene. After reflux overnight, the brown solution was poured into ice water and neutralized with dilute hydrochloric acid. The aqueous phase was extracted three times with ether, and the organic phases were combined and dried over anhydrous sodium sulfate. Diethyl ether was distilled off under reduced pressure, and 3.96 g of colorless li...
Embodiment 2
[0041] Embodiment 2: Based on the preparation of cycloheptyl-substituted DTBT and BDT alternating copolymers, the detailed route is shown in the appendix Figure 5 .
[0042] (1) 3-cycloheptylthiophene (TH- c 7) Synthesis of:
[0043] Reference TH- c The synthesis method of 5, add 1.2 g (50 mmol) magnesium and 1 grain of iodine into a 100 mL three-necked bottle, add 30 mL anhydrous ether through a syringe under the protection of argon, slowly add 8.85 g (50 mmol) bromocyclic Heptane, react until the magnesium disappears, place the reaction bottle in an ice bath, add 30 mg Pd(dppf)Cl 2 , and dropwise added 6.52 g (40 mmol) tribromothiophene. After reflux overnight, the brown solution was poured into ice water and neutralized with dilute hydrochloric acid. The aqueous phase was extracted three times with ether, and the organic phases were combined and dried over anhydrous sodium sulfate. Diethyl ether was distilled off under reduced pressure, and 4.76 g of colorless liquid ...
Embodiment 3
[0050] Embodiment 3: Based on the preparation of n-pentyl substituted DTBT and BDT alternating copolymers, the detailed route is attached Figure 6 .
[0051] (1) 3-n-pentylthiophene (TH- n 5) Synthesis of:
[0052] Reference TH- c The synthesis method of 5, add 1.2 g (50 mmol) magnesium and 1 iodine to a 100 mL three-necked bottle, add 30 mL anhydrous ether through a syringe under the protection of argon, and slowly add 7.55 g (50 mmol) bromo-n- Pentane, react until the magnesium disappears, place the reaction bottle in an ice bath, add 30 mg Ni(dppp)Cl 2 , and dropwise added 6.52 g (40 mmol) tribromothiophene. After reflux overnight, the brown solution was poured into ice water and neutralized with dilute hydrochloric acid. The aqueous phase was extracted three times with ether, and the organic phases were combined and dried over anhydrous sodium sulfate. Diethyl ether was distilled off under reduced pressure, and 4.63 g of colorless liquid was obtained by distillation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com