Preparation method of ertapenem single sodium salt
A technology of ertapenem and monosodium salt, which is applied in the field of penem medicine synthesis, can solve the problems of product quality decline, unfavorable large-scale production, and yield reduction, and achieve high product quality, reduced impact, and high product quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
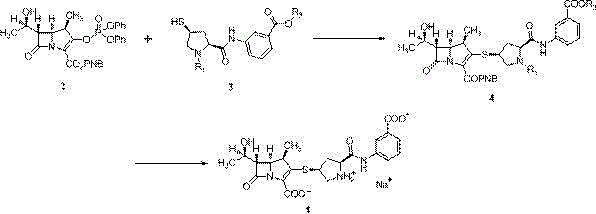
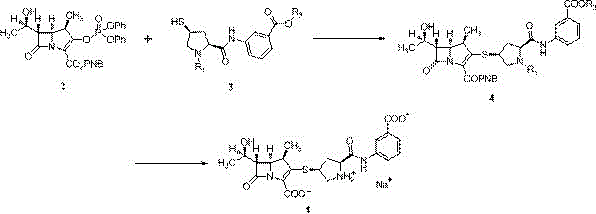
[0027] Example 1: Preparation of Ertapenem Sodium
[0028] In a 250ml clean and dry three-neck flask, add 20.0g MAP (33.6mmol) and 14.5g (32.6mmol) monoprotected ertapenem side chain, add 150ml N,N-dimethylformamide, stir and dissolve under nitrogen protection, then cool down to -30°C, add 11g of diisopropylethylamine (85.1mmol) dropwise, after dropping, control the temperature at -20~-30°C for 14~16h, take a sample and test, the product purity is 97.34%; after the reaction is completed, add 150ml to the reaction solution Tetrahydrofuran, transfer the reaction solution into a hydrogenation kettle, add 150ml of 5% sodium bicarbonate aqueous solution, then add 3.0g of palladium carbon, put together the kettle, ventilate and test the pressure, pressurize to 0.8MPa, react at 25-30℃ for 2h, stop the reaction , filtered, the filtrate was washed with ethyl acetate, the lower aqueous phase was adjusted to pH = 5.0 with acetic acid, washed with n-butanol, the lower aqueous phase was ad...
Embodiment 2
[0030] Example 2: Preparation of Ertapenem Sodium
[0031] In a 250ml clean and dry three-neck flask, add 20.0g MAP (33.6mmol) and 16.48g (37.0mmol) mono-protected ertapenem side chain, add 80ml N,N-dimethylformamide, stir to dissolve under nitrogen protection, then cool down to -25°C, add 9.1g of diisopropylethylamine (70.4mmol) dropwise, and react at -8~-15°C for 2~4h after dropping, take a sample and test, the purity of the product is 96.85%; after the reaction is completed, add 300ml of ethyl acetate and tetrahydrofuran mixed solution, the reaction solution was transferred to a hydrogenation kettle, 300ml of 5% sodium bicarbonate aqueous solution was added, and then 4.0g of palladium carbon was added, the kettle was put together, the pressure was tested by ventilation, and the pressure was increased to 1.2MPa. React at 25°C for 4 hours, stop the reaction, filter, separate the filtrate, adjust the pH of the lower aqueous phase to 7.0 with acetic acid, wash with ethyl acetat...
Embodiment 3
[0032] Example 3: Preparation of Ertapenem Sodium
[0033]In a 250ml clean and dry three-neck flask, add 20.0g MAP (33.6mmol) and 15.0g (33.7mmol) monoprotected ertapenem side chain, add 150ml acetonitrile, stir and dissolve under nitrogen protection, cool to -35°C, add dropwise 8.5 g diisopropylamine (84.0mmol), react at -15~-25℃ for 12~14h after dropping, take a sample and test, the product purity is 97.32%; after the reaction is completed, add 300ml tetrahydrofuran to the reaction solution, and the reaction solution is transferred to hydrogenation In the kettle, add 150ml of 2% sodium bicarbonate aqueous solution, then add 5.0g of palladium carbon, close the kettle, ventilate and test the pressure, pressurize to 1.5MPa, react at 10~15℃ for 6h, stop the reaction, filter, add ethyl acetate to the filtrate Wash the lower aqueous phase with acetic acid to adjust pH=6.5, wash with ethyl acetate, adjust the lower aqueous phase to pH=6.0, add 0.6 times the volume of ethyl acetate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com