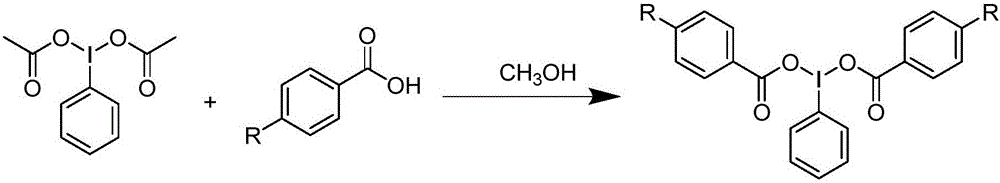
Preparation method and device for iodobenzene dibenzoate derivative
A technology of dibenzoic acid and iodobenzene diacetate is applied in the field of preparation of iodobenzene dibenzoate derivatives, can solve problems such as difficult removal, environmental pollution of chlorobenzene, lower reaction yield and the like, and achieves good reactivity and oxidation The effect of stability, simple process and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1, iodobenzene dibenzoate
[0021] Preparation of:
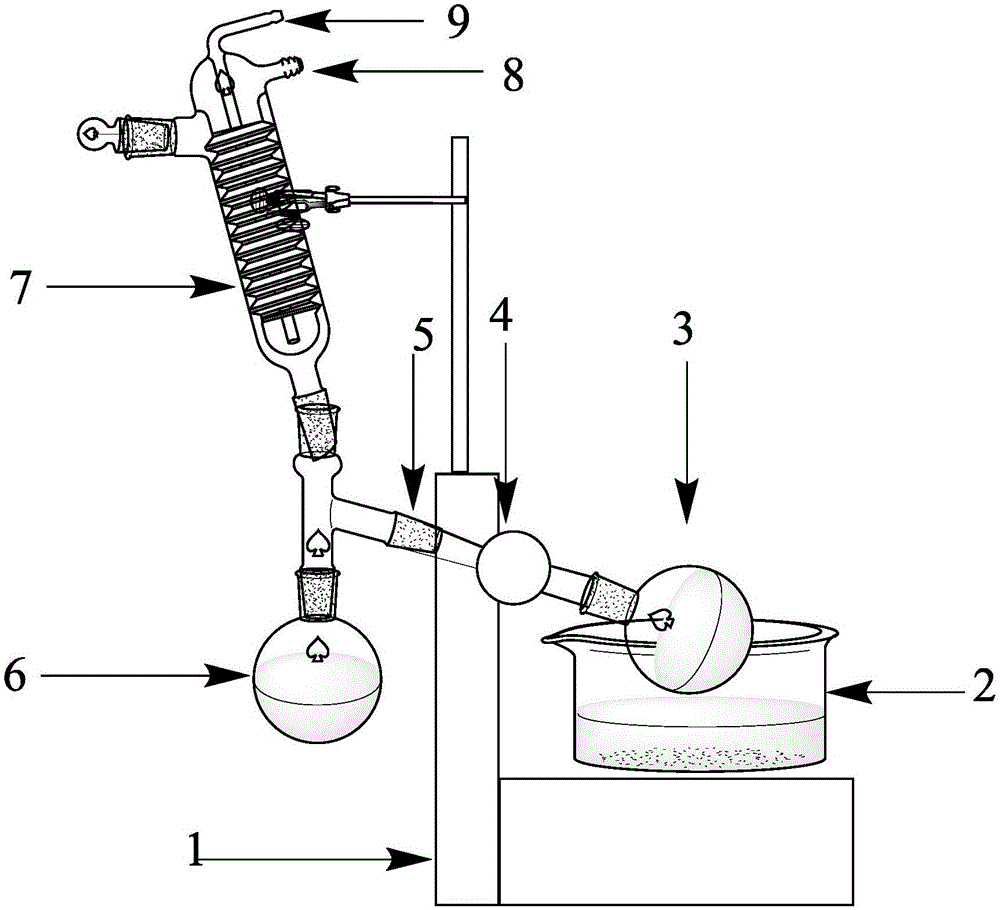
[0022] First add 6 mmol of iodobenzene diacetate, 12 mmol (2 equiv) of benzoic acid, and 5 ml of methanol as a solvent in a 25 mL round-bottomed flask until all benzoic acid and iodobenzene diacetate are dissolved in methanol; place the round-bottomed flask on a rotary evaporator Heat to 45°C and react for 0.5h; after the reaction, distill under reduced pressure and spin dry the solvent, and a large amount of white solid can be seen to precipitate; pour the solid obtained from the reaction liquid into a Buchner funnel, add 15ml of methanol to wash three times, and leave the remaining white solid in the air Dry; That is, iodobenzene dibenzoate. The yield was 86%.
[0023] H NMR 1 H NMR (400MHz, CDCl 3 ): δ7.37(t, J=7.64Hz, 4H), 7.47-7.57(m, 4H), 7.60-7.65(m, 1H), 7.93(d, J=7.64Hz, 4H), 8.24(d, J = 7.64Hz, 2H); 13 C NMR (100MHz, CDCl 3 ): δ122.3, 128.2, 130.1, 130.2, 131.0, 131.7, 132.5, 134.9, 171.3...
Embodiment 2
[0024] Embodiment 2, two (4-methoxybenzoic acid) iodobenzene
[0025] Preparation of:
[0026] In a 25mL round bottom flask, first add 6mmol of iodobenzene diacetate, 12mmol (2equiv) of 4-methoxybenzoic acid, and add 5ml of methanol as a solvent until all 4-methoxybenzoic acid and iodobenzene diacetate are dissolved in methanol; Place the round-bottomed flask on a rotary evaporator and heat it to 45°C for 0.5 hours; after the reaction, distill under reduced pressure and spin the solvent to dryness, and a large amount of white solids can be seen to precipitate; pour the solids obtained from the reaction solution into a Buchner funnel, and add 15ml of methanol was washed three times, and the remaining white solid was air-dried to obtain bis(4-methoxybenzoic acid)iodobenzene with a yield of 82%.
[0027] Bis(4-methoxybenzoic acid)iodophenyliodobenzenehydrogen NMR 1 H NMR (400MHz, CDCl 3 ): δ3.83(s,6H),6.85(d,J=8.88Hz,4H),7.53(t,J=7.84Hz,2H),7.59-7.63(m,1H),7.88(d,J= 8.84Hz,...
Embodiment 3
[0028] Embodiment 3, two (4-methylbenzoic acid) iodobenzene
[0029] Preparation of:
[0030] In the 25mL round bottom flask, add iodobenzene diacetate 6mmol earlier, 4-methoxybenzoic acid 12mmol (2equiv), add 5ml methyl alcohol as solvent until 4-methylbenzoic acid and iodobenzene diacetate are all dissolved in methanol; Place the round-bottomed flask on a rotary evaporator and heat it to 45°C for 0.5 hours; after the reaction, distill the solvent under reduced pressure and spin it dry, and a large amount of white solid can be seen to precipitate; pour the solid obtained from the reaction solution into a Buchner funnel, and add 15ml After washing with methanol three times, the remaining white solid was air-dried to obtain bis(4-methylbenzoic acid)iodobenzene with a yield of 85%.
[0031] H NMR 1 H NMR (400MHz, CDCl 3 ): δ2.37(s, 6H), 7.16(d, J=7.96Hz, 4H), 7.53(t, J=7.80Hz, 2H), 7.59-7.62(m, 1H), 7.82(d, J= 8.08Hz, 4H), 8.23(d, J=7.76Hz, 2H); 13 CNMR (100MHz, CDCl 3 )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com