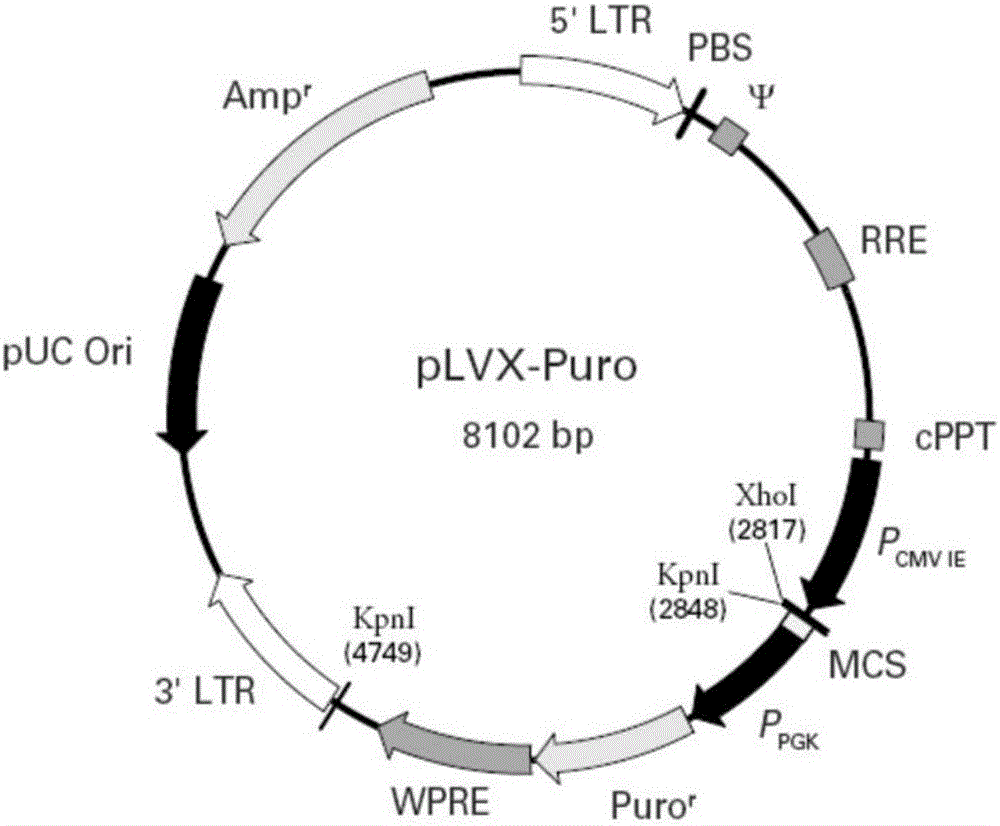
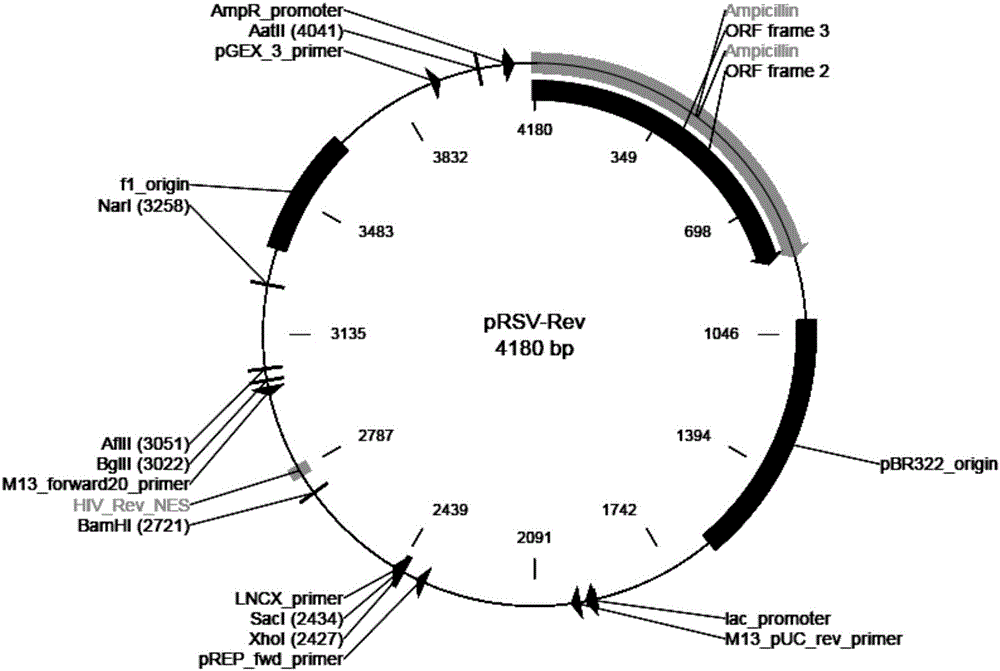
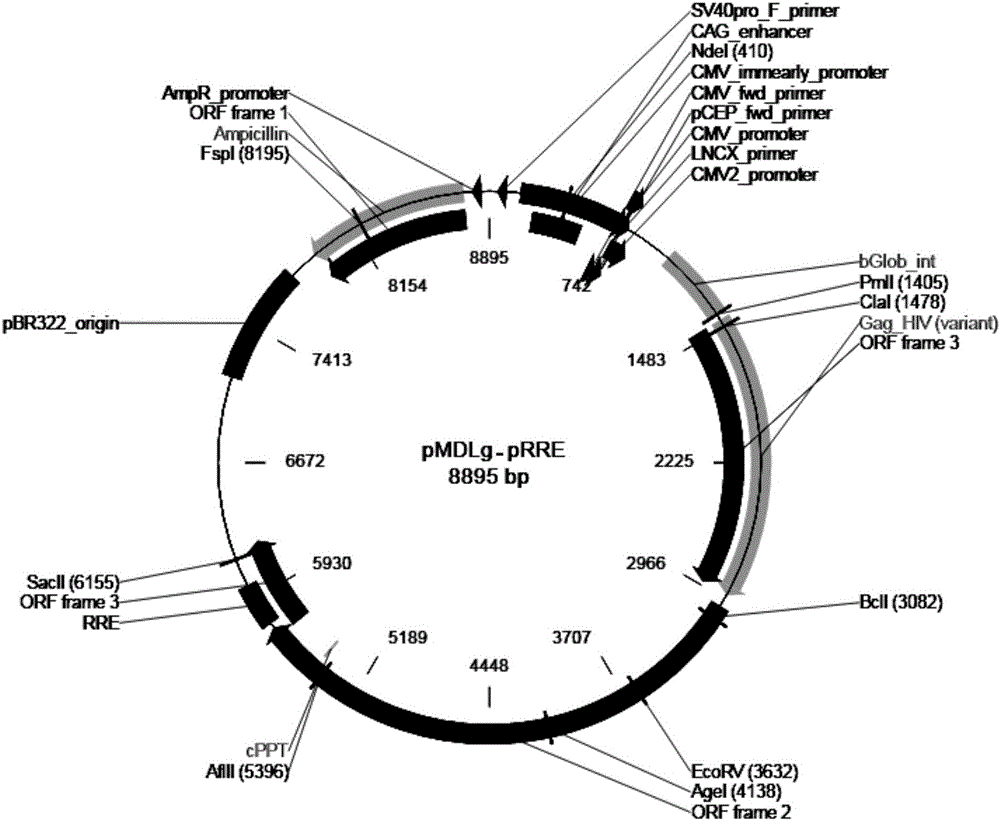
T1R3 gene overexpressed lentiviral vector and lentivirus, and construction methods thereof
A lentiviral vector and gene overexpression technology, applied in the field of molecular biology, can solve the problems of low success rate of cell lines and low transfection efficiency, and achieve the effect of promoting stable expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: T1R3 gene overexpression lentiviral vector construction.
[0045] 1. Artificially synthesize the T1R3 gene, the DNA of which is shown in SEQ ID NO.1.
[0046] 2. Use PrimeSTAR high-fidelity enzymes, use the artificially synthesized T1R3 gene as a template, and perform PCR amplification with the primers shown in Table 1:
[0047] Table 1
[0048]
[0049]
[0050] The PCR reaction system and conditions are shown in Table 2:
[0051] Table 2
[0052] Reagent
volume
template
1μL
Upstream primer T1R3-F (10μM)
1μL
Downstream primer T1R3-R (10μM)
1μL
Prime STAR Max (2x)
10 μL
wxya 2 o
7μL
total capacity
20 μL
[0053] The PCR program is shown in Table 3:
[0054] table 3
[0055]
[0056] 3. Agarose gel electrophoresis to detect whether the size of the PCR amplification product is consistent with the expectation, cut the target fragment band in the agarose gel electr...
Embodiment 2
[0071] Example 2: Packaging of T1R3 lentivirus
[0072] 1. Digest HEK293 cells in the logarithmic growth phase with trypsin, and the cell density is 5×10 6 re-inoculated in a cell culture dish with a diameter of 15 cm containing 25 mL of antibiotic-free DMEM medium containing 10% (v / v) FBS, at 37 °C, 50 mL / L CO 2 Culture in the incubator;
[0073] 2. Prepare four plasmid DNA solutions in the lentiviral packaging system:
[0074]
[0075] Add the above plasmid to sterile water to make up to 1800μL, then add CaCl 2 (2.5mol / L) solution 200μL, mix well, add 2000μL of 2×PBS buffered saline solution, and place at room temperature for 20-30min;
[0076] 3. Transfect when the cell density reaches 60%-70%. At this point, transfer the DNA solution and calcium phosphate mixture prepared in step 2 to the cell culture dish containing monolayer cells obtained in step 1, mix well, discard the culture solution after culturing for 12 h, and wash the cells with 15 mL of PBS solution for ...
Embodiment 3
[0083] Example 3: Cell Transfection and Screening
[0084] 1. Cultivate HEK293 cells in a 6-well cell culture plate. After the cells are completely adherent and confluent to 40%-50%, the cells can be transfected; before transfection, replace the cells in each well with 500 μL of fresh DMEM for complete culture Base, and 1 μL of polybrene (polybrene) was added to each well, and placed in an incubator for 1 h.
[0085] 2. Add 5 μL of the T1R3 lentivirus solution obtained in Example 2 to the two wells of the above-mentioned 6-well cell culture plate, and add 10 μL of the empty lentivirus solution to the cells in one well (the empty lentivirus solution and the T1R3 lentivirus solution The only difference is that it does not contain the T1R3 gene, and the rest of the preparation methods are the same), and the other well is used as a blank cell control. 2 , 95% relative humidity incubator culture
[0086] 3. After 6 hours of transfection, suck away the medium in the well, and wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com