3,5-dimethylthio-2,4-bis(4,4'-dinitroazophenylaminoazo)toluene, preparation method and applications thereof
A dinitroazophenylaminoazo group and dimethylthio group technology, applied in the field of bistriazene compounds, can solve the problems of unsatisfactory selectivity and low sensitivity of reagents, so as to increase electron mobility and improve sensitivity , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
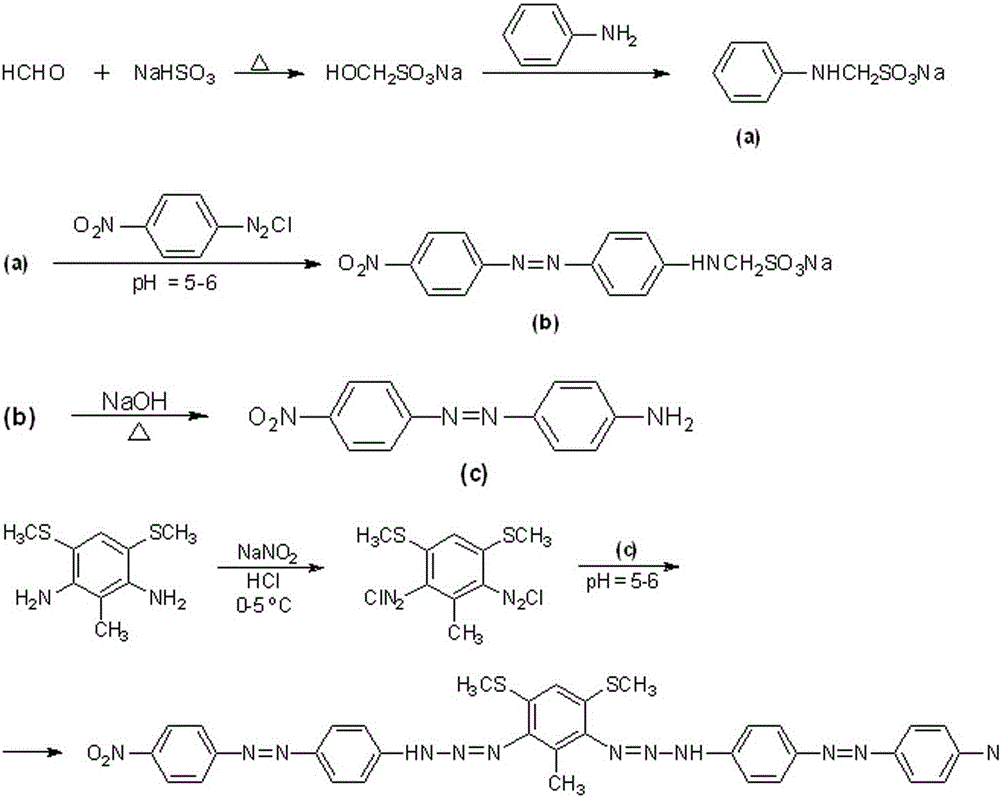
[0037] A kind of 3,5-dimethylthio-2-bis(4,4´-dinitroazophenylaminoazo)toluene, abbreviated as DMSNZAZB, the molecular structure formula is as follows:
[0038] .
[0039] The preparation method of 3,5-dimethylthio-2-two (4,4'-dinitroazophenylaminoazo) toluene comprises the following steps, such as figure 1 Shown:
[0040] (1) Synthesis of 4-nitro-4´-aminoazobenzene
[0041] Add 10.4 g (0.1 mol) NaHSO to the beaker 3 , then add 38 mL of water and 8 mL of 40 % (0.1 mol) formaldehyde by volume fraction, stir in a water bath at 58-63 ℃ for 40 min, add 6.7 g (0.07 mol) of aniline, and react for 1.5 h to obtain light yellow anilinomethyl Sodium sulfonate clarifies the solution, and another 9.8 g (0.07 mol) p-nitroaniline is dissolved in 30 mL HCl (1+1, volume ratio), and 20 mL containing 4.9 g NaNO 2 After adding the aqueous solution, react for 30 min to obtain yellow diazonium salt for subsequent use;
[0042] Under cooling in an ice-water bath, add this diazonium salt to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com