Light-responding methyl methacrylate azo polymer and synthetic method thereof
A technology of methyl methacrylate and methyl methacrylate is applied in the field of polymer synthesis and preparation, and achieves the effects of high yield, mild reaction conditions and fast photoresponsivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
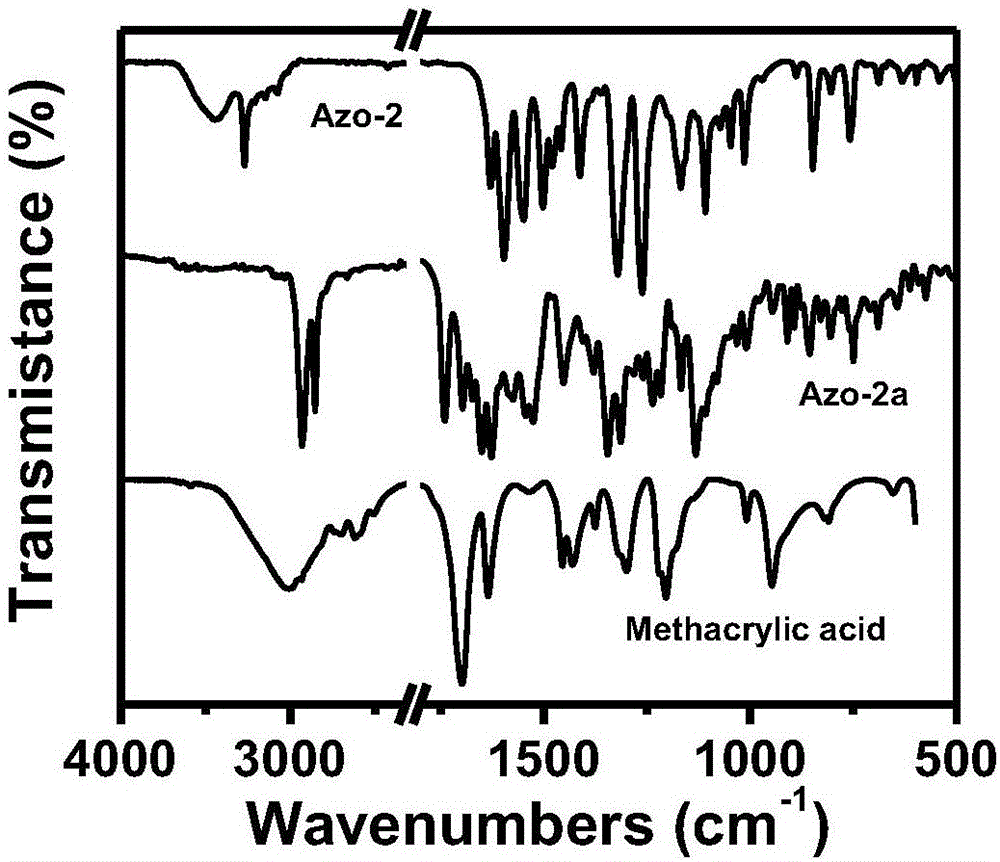
[0035] (1) Synthesis of azo monomer: at 0°C, with dichloromethane as solvent, slowly drop 0.01mol / L 4-(4-nitrophenylazo)-1-naphthol solution into toluene In base acrylic acid, the molar mass ratio of 4-(4-nitrophenylazo)-1-naphthol to methacrylic acid is 2:1; add 4-dimethylaminopyridine (DMAP) and dicyclohexyl carbon to the system Diimine (DCC), the molar mass ratio of DMAP and DCC is 1:5. First react at 0°C for 2 hours, then raise the temperature to 25°C, and continue the reaction for 12 hours to obtain the azo monomer. The structural formula is:
[0036]
[0037] (2) Synthesis of methyl methacrylate-based azo polymers: carry out polycondensation reaction between methyl methacrylate and the synthesized azo monomer in a molar mass ratio of 1:10, and the addition amount of the initiator AIBN is 1% of the amount of methyl acrylate added. First add methyl methacrylate and AIBN into the container, blow nitrogen at 25°C to remove oxygen in the system; then slowly add the azo ...
Embodiment 2
[0039] (1) Synthesis of azo monomer: at 0°C, with dichloromethane as solvent, slowly drop 0.1mol / L 4-(4-nitrophenylazo)-1-naphthol solution into toluene In the base acrylic acid, the molar mass ratio of 4-(4-nitrophenylazo)-1-naphthol to methacrylic acid is 1:1. Add 4-dimethylaminopyridine (DMAP) and dicyclohexylcarbodiimide (DCC) to the system, the molar mass ratio of DMAP and DCC is 1:10; first react at 0°C for 2 hours, then heat up to 25°C , continue to react for 12 hours to obtain azo monomer. The structural formula is:
[0040]
[0041] (2) Synthesis of methyl methacrylate azo polymer: methyl methacrylate and the azo monomer synthesized in step 1 are subjected to polycondensation reaction in a ratio of 1:15 by molar mass ratio, the addition amount of initiator AIBN 3% of the amount of methyl methacrylate added. First, add methyl methacrylate and AIBN into the container, blow nitrogen at 25°C to remove oxygen in the system; then slowly add the azo monomer prepared in...
Embodiment 3
[0048] (1) Synthesis of azo monomer: at 0°C, with dichloromethane as solvent, slowly drop 1mol / L 4-(4-nitrophenylazo)-1-naphthol solution into methyl In acrylic acid, the molar mass ratio of 4-(4-nitrophenylazo)-1-naphthol to methacrylic acid is 1:5. Add 4-dimethylaminopyridine (DMAP) and dicyclohexylcarbodiimide (DCC) to the system, the molar mass ratio of DMAP and DCC is 1:20; first react at 0°C for 5 hours, then heat up to 25°C , continue to react for 24 hours to obtain azo monomer. The structural formula is:
[0049]
[0050] (2) Synthesis of methyl methacrylate azo polymer: carry out polycondensation reaction with methyl methacrylate and the azo monomer synthesized in step 1 in a molar mass ratio of 1:100, the addition amount of initiator AIBN 5% of the amount of methyl methacrylate added. First add methyl methacrylate and AIBN into the container, blow nitrogen at 25°C to remove oxygen in the system; then slowly add the azo monomer prepared in step 1 to the system d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com