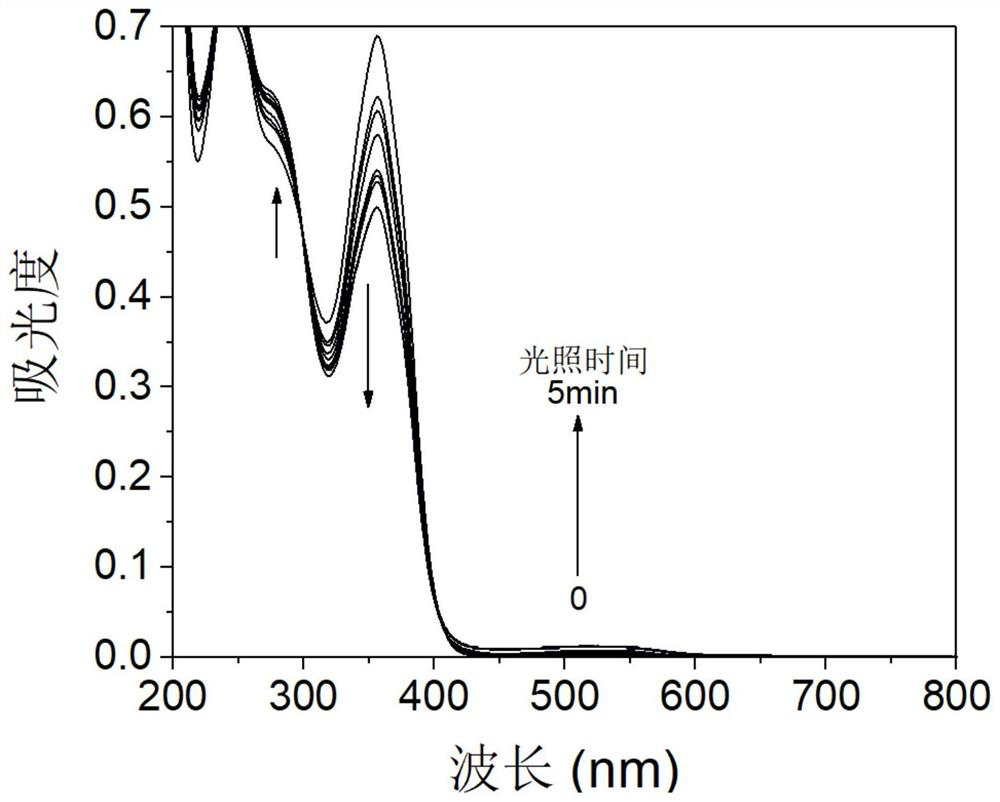
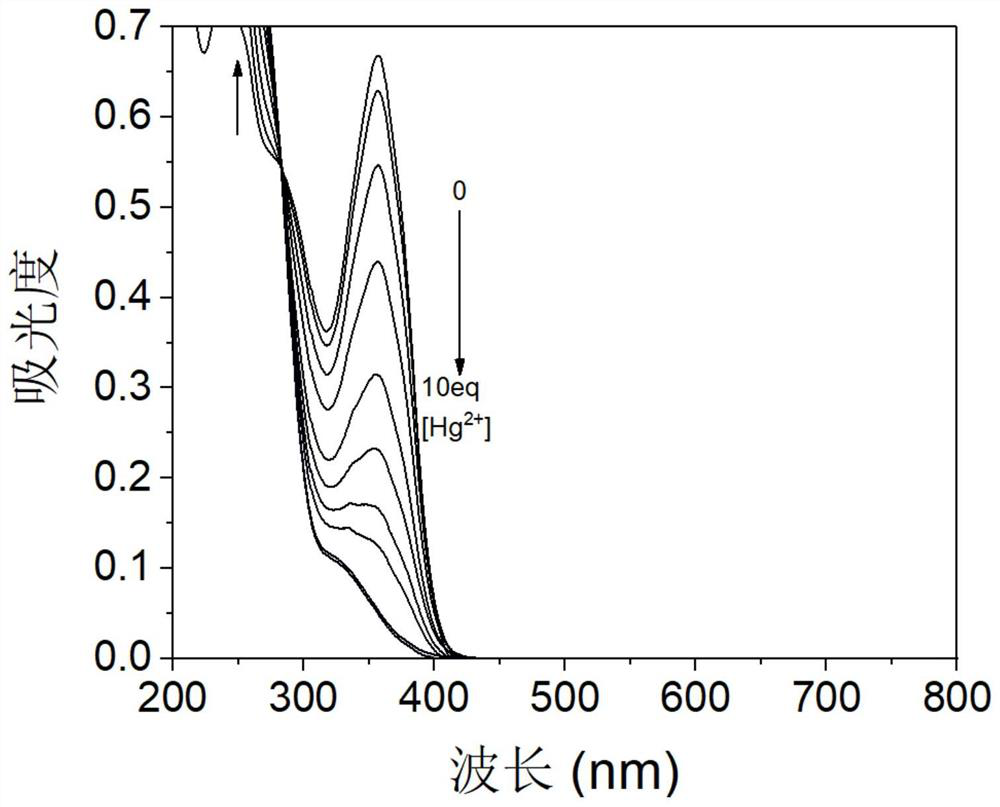
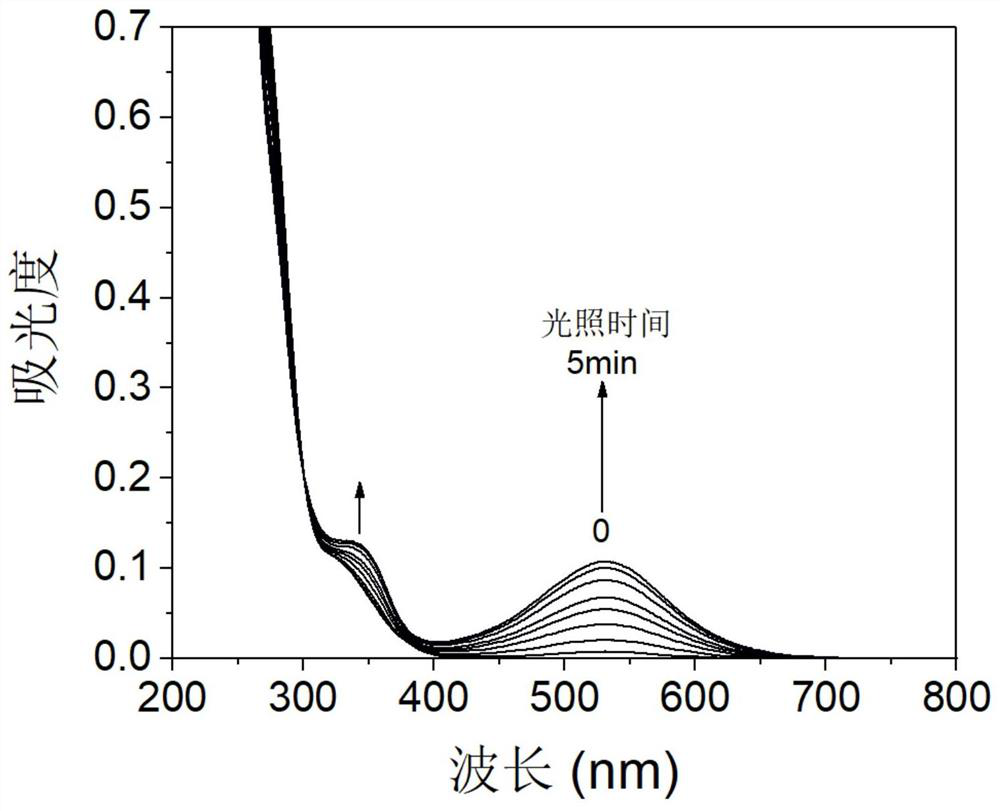
A kind of organic photochromic material and its preparation method and application
A photochromic material and organic technology, applied in the field of color-changing materials, to achieve the effects of few synthesis steps, excellent fatigue resistance and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Synthesis of organic photochromic material P1
[0038] Synthesis of Compound P1
[0039]
[0040] The preparation method of 1-(5-chloro-2-methyl-3-thienyl)-2-(5-formyl-2-methyl-3-thienyl)cyclopentene (compound 1) refers to literature (H .Tian, et al., Tetrahedron, 2011, 67, 915-921), 4-aminoantipyrine (compound 2) can be purchased directly.
[0041] In the dark, at room temperature, sequentially add 1-(5-chloro-2-methyl-3-thienyl)-2-(5-formyl-2-methyl-3-thienyl) into a 50mL single-necked bottle ) cyclopentene (0.225g, 0.699mmol), 4-aminoantipyrine (0.142g, 0.7mmol), 15mL ethanol, slowly warming up to 78°C, stirring under reflux for 6h, cooling to room temperature naturally, filtering , the filter cake was washed with ethanol and dried to obtain 0.201 g of golden yellow powder solid P1 with a yield of 59.2%. 1H NMR (400MHz, Chloroform-d) δ1.84(s,3H),2.00-2.07(m,5H),2.42(s,3H),2.70-2.79(m,4H),3.11(s,3H), 6.59(s,1H),7.01(s,1H),7.31(t,J=8.0Hz,1H),7.39(d,J=8.0Hz,...
Embodiment 2
[0047] Synthesis of organic photochromic materials:
[0048] In the dark, at room temperature, sequentially add 1-(5-chloro-2-methyl-3-thienyl)-2-(5-formyl-2-methyl-3-thienyl) into a 50mL single-necked bottle ) cyclopentene (0.225g, 0.699mmol), 4-aminoantipyrine (0.284g, 1.4mmol), 42mL toluene, slowly warming up to 65°C, stirring under reflux for 24h, cooling to room temperature naturally, filtering , The filter cake was washed with ethanol and dried to obtain a golden yellow powdery solid.
Embodiment 3
[0050] Synthesis of organic photochromic materials:
[0051] In the dark, at room temperature, sequentially add 1-(5-chloro-2-methyl-3-thienyl)-2-(5-formyl-2-methyl-3-thienyl) into a 50mL single-necked bottle ) cyclopentene (0.45g, 1.4mmol), 4-aminoantipyrine (0.426g, 2.1mmol), 10.5mL toluene, slowly warming up to 110°C, stirring for 1h under reflux, and cooling to room temperature naturally, After filtering, the filter cake was washed with ethanol and dried to obtain a golden yellow powdery solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com