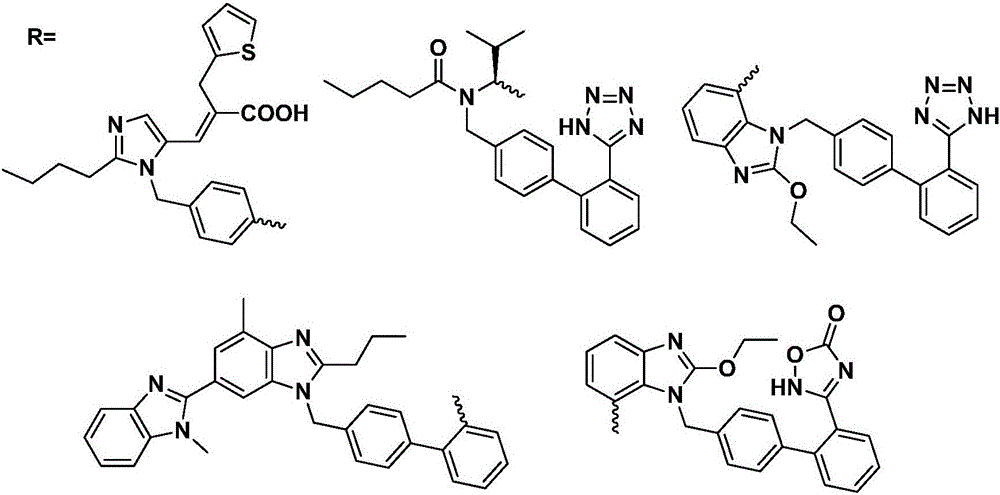
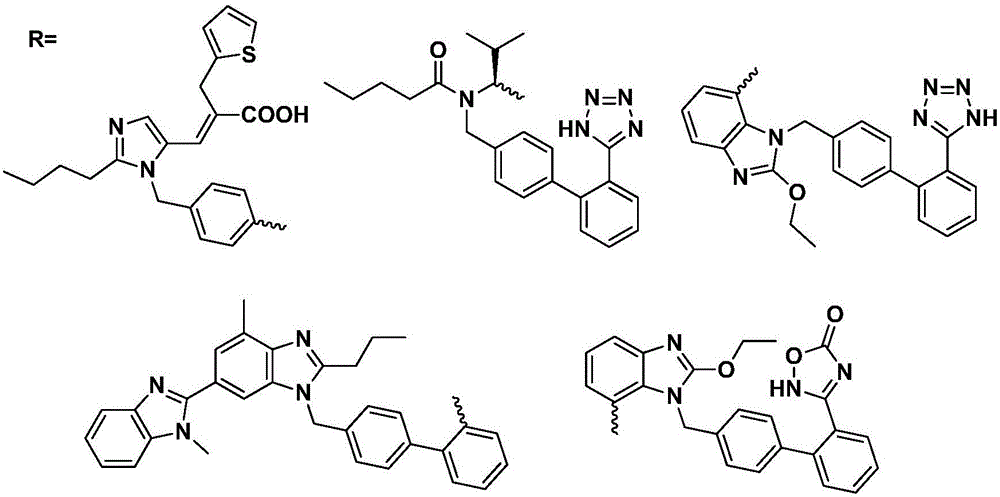
Preparation method and use of 9-bit-substituted double functional group berberine derivatives
A derivative, the technology of berberine, which is applied in the field of food and pharmacy, can solve the problems of low fat solubility, low water solubility of berberine hydrochloride, and influence on systemic therapeutic effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] a) Synthesis of Berberine
[0021] Add 7.4g of berberine to a 250mL round flask, heat at 190-200°C for about 30min at a vacuum of 20-30mmHg, the yellow solid gradually turns dark red, cool to room temperature in a vacuum desiccator, and purify by silica gel column chromatography. Obtained 4.7 g of dark red powder with a yield of 75%.
[0022] b) Synthesis of 9-O-3-hydroxyl-ethyl berberine hydrobromide
[0023] Add 3.2g (10mmol) of berberine into a 25mL round bottom flask, add 30mL of DMF to dissolve, heat at 70°C, add 4.4g (20mmol) of 2-bromoethanol, follow the reaction by TLC, add 100mL of anhydrous ether after the reaction is complete, and precipitate The solid was filtered and purified by silica gel column chromatography to obtain 3.3 g of 9-O-3-hydroxy-ethyl berberine hydrobromide with a yield of 76%
[0024] c) Synthesis of eprosartan-9-O-acetate berberine
[0025] Add 425mg (1mmol) eprosartan, 20ml dichloromethane, 178mg (1.1mmol) CDI to a 25mL round bottom fla...
Embodiment 2
[0027] a) Synthesis of Berberine
[0028] Same as a) in Example 1
[0029] b) Synthesis of 9-O-3-hydroxyl-ethyl berberine hydrobromide
[0030] Same as b) in Example 1
[0031] c) Synthesis of valsartan-9-O-acetate berberine
[0032] Add 436mg (1mmol) valsartan, 20ml dichloromethane, 178mg (1.1mmol) CDI to a 25mL round bottom flask, stir for 10min, then add 446mg 9-O-3-hydroxy-ethylberberine hydrobromide (1mmol), stirred at room temperature, followed by TLC reaction, after the reaction was complete, 20ml of water was added, stirred, extracted, the organic phase was dried, filtered, concentrated under reduced pressure, and purified by silica gel column chromatography to obtain valsartan-9-O-acetic acid Ester berberine 659mg, yield 86%. ESI-MS(M+H) + m / z calcd C 45 h 47 N 6 o 7 + for784.35found784.35.
Embodiment 3
[0034] a) Synthesis of Berberine
[0035] Same as a) in Example 1
[0036] b) Synthesis of 9-O-3-hydroxyl-ethyl berberine hydrobromide
[0037] Same as b) in Example 1
[0038] c) Synthesis of candesartan-9-O-acetate berberine
[0039] Add 440mg (1mmol) candesartan, 20ml dichloromethane, 178mg (1.1mmol) CDI to a 25mL round bottom flask, stir for 10min, then add 9-O-3-hydroxy-ethylberberine hydrobromide 446 mg (1 mmol), stirred at room temperature, TLC followed the reaction, after the reaction was complete, 20 ml of water was added, stirred, extracted, the organic phase was dried, filtered, concentrated under reduced pressure, and purified by silica gel column chromatography to obtain candesartan-9-O- Acetate berberine 694mg, yield 88%. ESI-MS(M+H) + m / z calcd C 45 h 38 N 7 o 7 + for789.28found789.28.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com