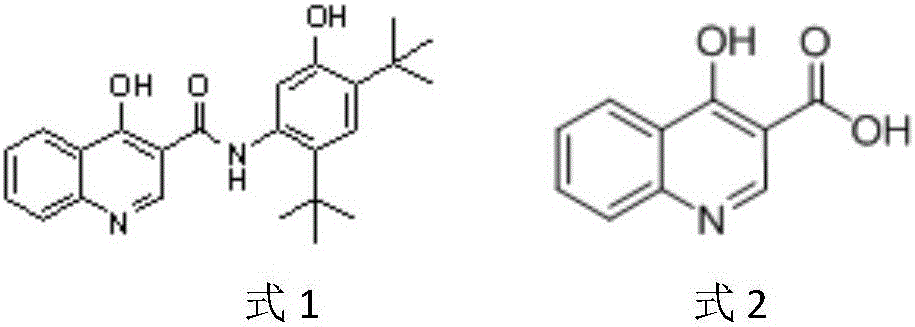
Preparation method of 4-hydroxyquinoline-3-carboxylic acid
A technology of hydroxyquinoline and o-nitrobenzoic acid, applied in the direction of organic chemistry, can solve the problems of high production cost and environmental pollution of 4-hydroxyquinoline-3-carboxylic acid, achieve good application prospects, reduce costs, avoid Effect of poorly soluble impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
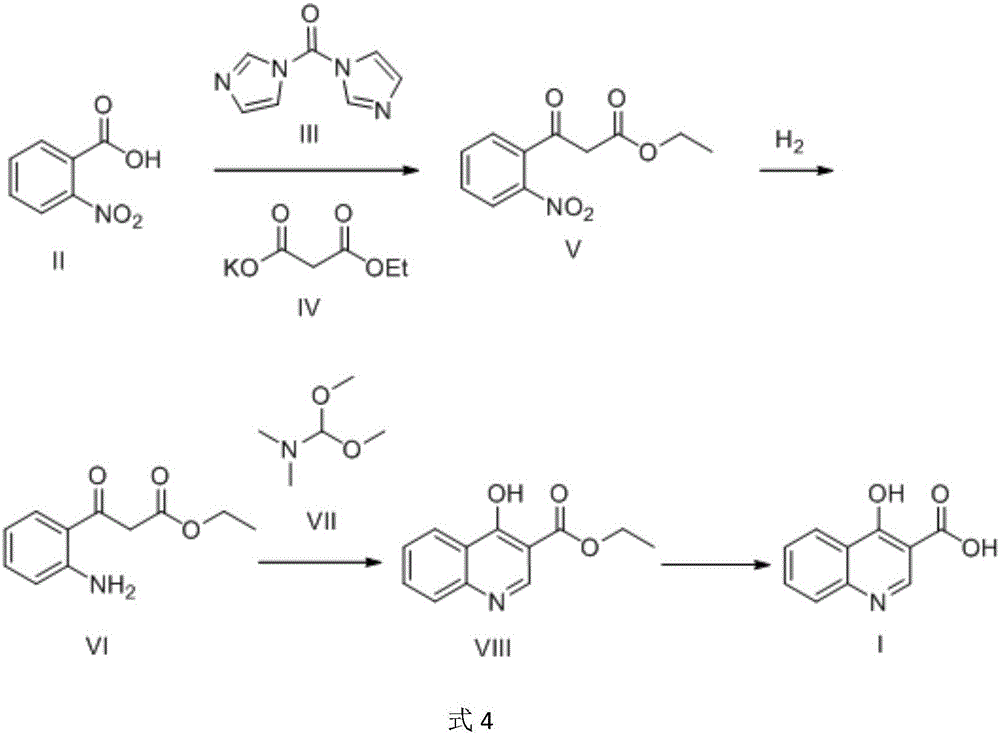
[0035] Example 1 Preparation of 3-(2-nitrophenyl)-3-oxopropionic acid ethyl ester (1)
[0036] A: Mix o-nitrobenzoic acid (3.0 g, 18 mmol) and 30 mL of acetonitrile, stir at 20° C., add N,N-carbonyldiimidazole (3.2 g, 19.8 mmol) in batches, and react for 4 hours. The solution is yellowish and clear.
[0037] B: Take a three-necked flask and add monoethyl malonate potassium salt (15.3 g, 90 mmol) into 60 mL of acetonitrile, and stir. Install the thermometer, use about 15min to MgCl 2 (1.85 g, 19.44 mmol) was added in portions. The temperature of the reaction solution rose slightly. After the addition, it was stirred at 35°C for 30min, then cooled to 25°C, and triethylamine (5.46g, 54mmol, 7.52mL) was slowly added dropwise under stirring. With the addition of triethylamine, the reaction solution became thicker and appeared White lumps. Stir for 30 min after addition.
[0038] C: Add the reaction solution in A to B, and the temperature rises slightly. Then it was stirred a...
Embodiment 2
[0041] Example 2 Preparation of ethyl 3-(2-aminophenyl)-3-oxopropionate (1)
[0042] Dissolve ethyl 3-(2-nitrophenyl)-3-oxopropionate (2.4 g, 0.01 mol) in methanol (50 mL), and add 0.5 g of catalyst Raney nickel; The reaction was stirred at 20°C for 8 hours, the catalyst was filtered out through diatomaceous earth, and concentrated to obtain 2.0 g of ethyl 3-(2-aminophenyl)-3-oxopropionate with a yield of 96% as a brown liquid.
[0043] 1 H NMR (400MHz, CDCl 3 ):δ=1.31(t,J=7.2Hz,3H),3.44(s,2H),4.24(q,J=7.2Hz,2H),6.68(m,2H),7.30(m,1H),7.86 (m,1H),8.77(brs,2H).MS(ESI):m / z=207.0[M–H] – .
Embodiment 3
[0044] The preparation of embodiment 3 4-hydroxyquinoline-3-formic acid ethyl ester (1)
[0045] 3-(2-aminophenyl)-3-oxopropanoic acid ethyl ester (1.8g, 0.0087mol) was dissolved in toluene (30mL), and N,N-dimethylformamide dimethyl acetal was added thereto (2.9g, 0.025mol), reacted at reflux temperature for 8h; the reaction solution was cooled to 20°C, the precipitated solid was filtered and washed with a mixture of equal volumes of ethanol and water (2mLx2 times), dried at 50°C, washed with 15mL ethyl acetate Recrystallize and purify the mixed liquid with an equal volume of petroleum ether to obtain 1.3 g of ethyl 4-hydroxyquinoline-3-carboxylate, with a yield of 70%, as an off-white solid.
[0046] 1 H NMR (400MHz, DMSO-d 6 ):δ=1.29(t,J=7.2Hz,3H),4.22(q,J=7.2Hz,2H),7.42(m,1H),7.62(m,1H),7.71(m,1H),8.16 (m,1H),8.55(s,1H),12.31(s,1H).MS(ESI):m / z=218.2[M+H] + .
[0047] HPLC: Column: InertSustain C18 (250mm×4.6mm×5μm); Detection wavelength: 220nm; Flow rate: 0.8mL / min; Co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com