Pemetrexed disodium injection solution and preparation method thereof
A technique for pemetrexed disodium and injections, which is applied in the field of pemetrexed disodium injections and its preparation, can solve the problems of failure to achieve targeted drug delivery, slow down of elimination speed, etc., and achieve simple and reliable production technology, including The effect of high sealing rate and relaxed storage conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
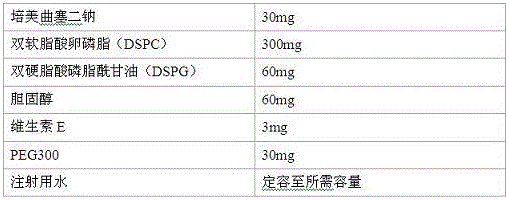
[0030] Prescription
[0031]
[0032] The preparation process is as follows:
[0033] Dissolve pemetrexed disodium, phospholipids, cholesterol and vitamin E in methanol according to the formula and mix them evenly; use a rotary evaporator to remove the methanol in the solution under reduced pressure to form a lipid film; dissolve PEG300 in 10ml of water for injection, and mix with water The lipid film was hydrated, and the hydration temperature was 40°C to obtain the pemetrexed disodium liposome suspension; after the hydration was complete, the liposomes were prepared with a high-pressure homogenizer until the average particle size was 130 ± 10nm, and the liposomes were Particle size and uniformity are detected with a multi-angle nanoparticle analyzer; the concentration that is constant with water for injection and adjusted to is a 2.0mg / ml solution, and the pemetrexed disodium liposome suspension is micronized with a 0.22 micron aperture. Filter and sterilize through the ...
Embodiment 2
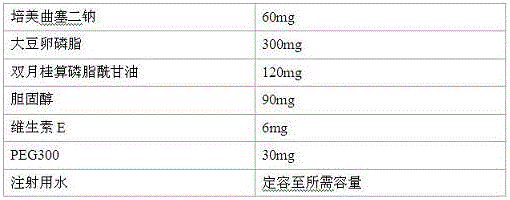
[0035] Prescription
[0036]
[0037] The preparation process is as follows:
[0038] Dissolve pemetrexed disodium, phospholipids, cholesterol and vitamin E in methanol according to the formula and mix them evenly; use a rotary evaporator to remove the methanol in the solution under reduced pressure to form a lipid film; dissolve PEG300 in 10ml of water for injection, and mix with water The lipid film was hydrated, and the hydration temperature was 30°C to obtain the pemetrexed disodium liposome suspension; after the hydration was complete, the liposomes were prepared with a high-pressure homogenizer until the average particle size was 120 ± 10nm, and the liposomes were Particle size and uniformity are detected with a multi-angle nanoparticle analyzer; the concentration that is constant with water for injection and adjusted to is a 2.0mg / ml solution, and the pemetrexed disodium liposome suspension is micronized with a 0.22 micron aperture. Filter and sterilize through the po...
Embodiment 3
[0040] Prescription
[0041]
[0042] The preparation process is as follows:
[0043] Dissolve pemetrexed disodium, phospholipids, cholesterol and vitamin E in methanol according to the formula and mix them evenly; use a rotary evaporator to remove the methanol in the solution under reduced pressure to form a lipid film; dissolve PEG300 in 10ml of water for injection, and mix with water The lipid film was hydrated, and the hydration temperature was 50°C to obtain the pemetrexed disodium liposome suspension; after the hydration was complete, the liposomes were prepared with a high-pressure homogenizer until the average particle size was 110 ± 10nm, and the liposomes were Particle size and uniformity are detected with a multi-angle nanoparticle analyzer; the concentration that is constant with water for injection and adjusted to is a 2.0mg / ml solution, and the pemetrexed disodium liposome suspension is micronized with a 0.22 micron aperture. Filter and sterilize through the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com