Method for preparing chlorzoxazone
A technology of chlorzoxazone and nitrophenol, which is applied in the field of chemical drug synthesis, can solve the problems of difficult industrial production, easy to be oxidized and discolored, and many solid wastes, so as to avoid oxidative deterioration, reduce residence time, and increase overall yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
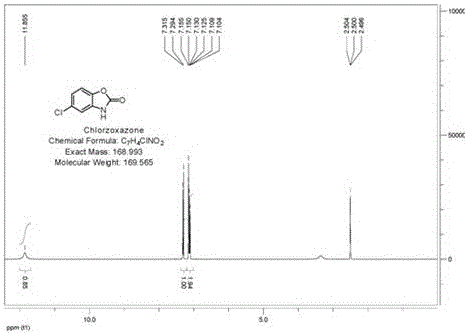
[0039] Example 1: Preparation of Chlorzoxazone
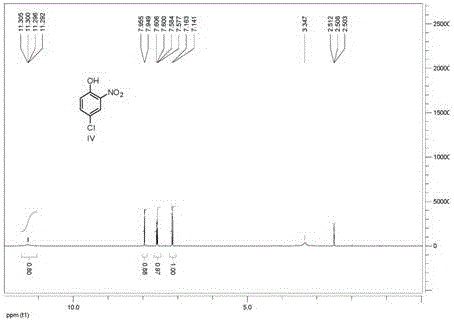
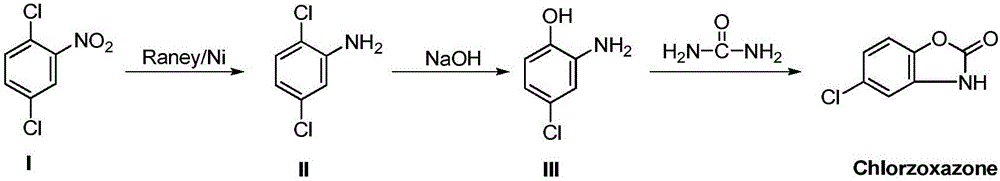
[0040] Add 50-100mL methanol, 1.0g Raney / Ni, 10g 4-chloro-2-nitrophenol into a 350mL autoclave, seal the reactor, start stirring, replace the air in the reaction system with hydrogen, and keep the hydrogen pressure 0.1~ 0.2MPa, room temperature reaction, chromatographic tracking reaction.
[0041] After the reaction, under the protection of nitrogen, filter to remove Raney / Ni. Add 10-30 mL of water to the filtrate, and distill off methanol. Add 5-7 mL of 36% concentrated hydrochloric acid and 0.5 g of activated carbon to the residual liquid, raise the temperature to 50°C, stir for half an hour, filter, and wash with water.
[0042] Add 8.6g of urea to the filtrate, heat to reflux for 1 hour, add concentrated hydrochloric acid to control the reaction liquid to be acidic, and continue to reflux for three hours. During this process, add concentrated hydrochloric acid to ensure the acidity of the reaction liquid Afterwards, reflux the r...
Embodiment 2
[0044] Example 2: Preparation of Chlorzoxazone
[0045] Add 200-400mL methanol, 4.0g Raney / Ni, 40g 4-chloro-2-nitrophenol to a 2L autoclave, seal the reactor, start stirring, replace the air in the reaction system with hydrogen, and keep the hydrogen pressure 0.1~ 0.2MPa, room temperature reaction. After the reaction is complete, protect with nitrogen and filter to remove Raney / Ni. 40-120 mL of water was added to the filtrate, and methanol was distilled off. Add 20-30 mL of 36% concentrated hydrochloric acid and 2 g of activated carbon to the residual liquid, raise the temperature to 50°C, stir for half an hour, filter, and wash with water. Add 34.4g of urea to the filtrate, heat to reflux for 1 hour, add concentrated hydrochloric acid to control the reaction liquid to be acidic, and continue to reflux for three hours. During this process, continuously add concentrated hydrochloric acid to ensure the acidity of the reaction liquid Afterwards, reflux the reaction overnight. Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com