Support metal catalyst and method for synthesizing ammonia using same catalyst
A technology for supporting metals and catalysts, applied in metal/metal oxide/metal hydroxide catalysts, nitrogen-metal/silicon/boron binary compounds, chemical instruments and methods, etc., can solve the problem of easy oxidation, instability, etc. problem, to achieve the effect of efficient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] 3 N 2 Preparation of >
[0083] 1 g of commercially available reagent Ca 3 N 2 Powder (BET surface area approx. 1m 2 g -1 ) in the glove box of Ar environment with 0.042g of Ru 3 (CO) 12 The powders are mixed and packed into vacuumed quartz glass tubes. While rotating the sample contained in the glass tube, it was heated at a temperature of 250° C. for 15 hours. Ca loaded with 2 wt% Ru metal particles was thus prepared 3 N 2 catalyst. The catalyst has a BET surface area of about 1 m 2 g -1 , there is almost no change in the BET surface area after loading. Ru dispersion (%) measured by CO adsorption method was 3.0.
[0084]
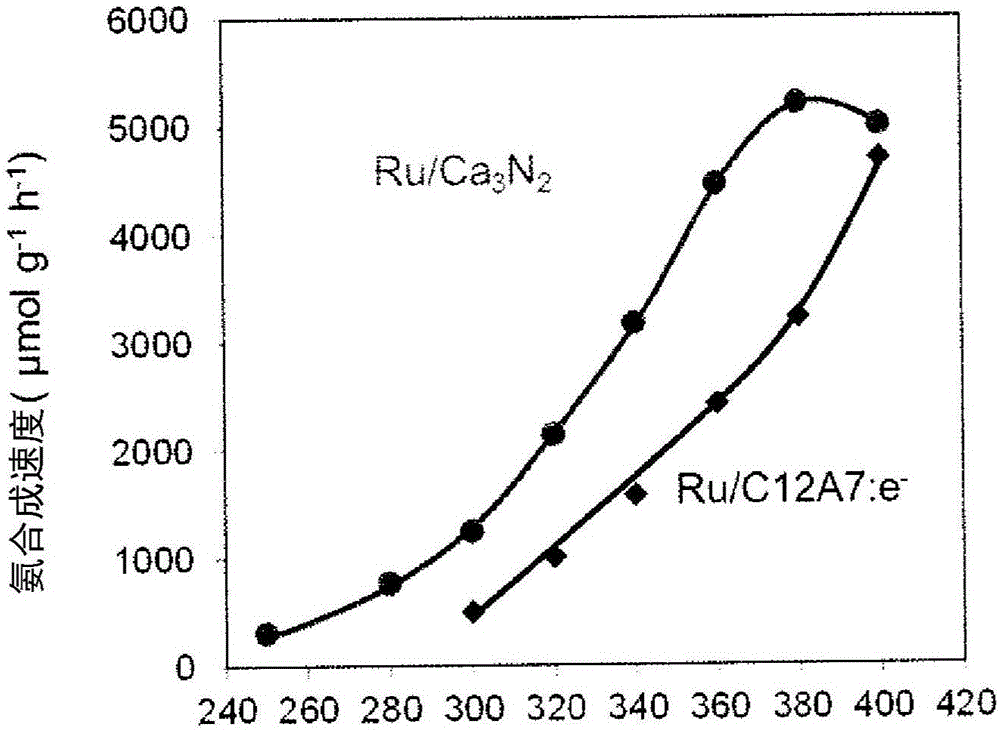
[0085] Nitrogen (N 2 ) and hydrogen (H 2 ) react to form ammonia (NH 3 ) synthesis reaction. When the reaction temperature is 400°C, put 0.2g of the catalyst prepared by the above method into a glass tube, and when the reaction temperature is 340°C, put 0.1g of the above catalyst into the glass tube, and use a fixed-bed flow-thr...
Embodiment 2
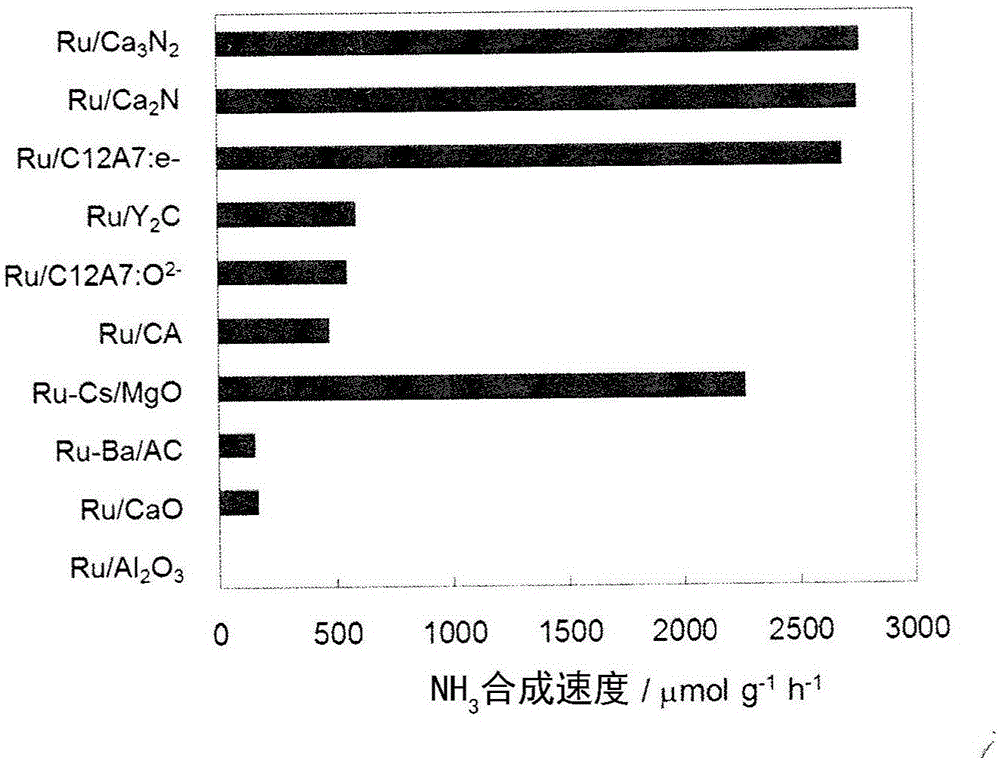
[0087] In addition to using Ca 2 N (BET surface area is 1m 2 g -1 ) instead of Ca in Example 1 3 N 2 In addition, the same method as in Example 1 was used to prepare 2wt% Ru / Ca 2 N catalyst, under the same conditions as in Example 1, the ammonia synthesis reaction was implemented. The rate of ammonia synthesis at 400°C is figure 1 As shown, it is 2750μmolg -1 h -1 . The rate of ammonia synthesis at 340°C is shown in Table 1, which is 3386 μmolg -1 h -1 . TOF (×10 -3 the s -1 ) is 171.2.
Embodiment 3
[0089] In addition to using Ca(NH 2 ) 2 (BET surface area is 120m 2 g -1 ) instead of Ca in Example 1 3 N 2 In addition, the same method as in Example 1 was used to prepare 2wt% Ru / Ca (NH 2 ) 2 Catalyst, implement ammonia synthesis reaction under the same conditions as in Example 1. The rate of ammonia synthesis at 340°C is shown in Table 1, which is 2118 μmolg -1 h -1 . TOF (×10 -3 the s -1 ) is 3.2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com