Industrial preparation method of leuprolide acetate
A technology for leuprolide and amino acids, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as poor product quality stability, racemization, serious environmental pollution, etc., and reduce the possibility of racemization Sexuality and other side effects, suppression of by-products, and safety-enhancing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
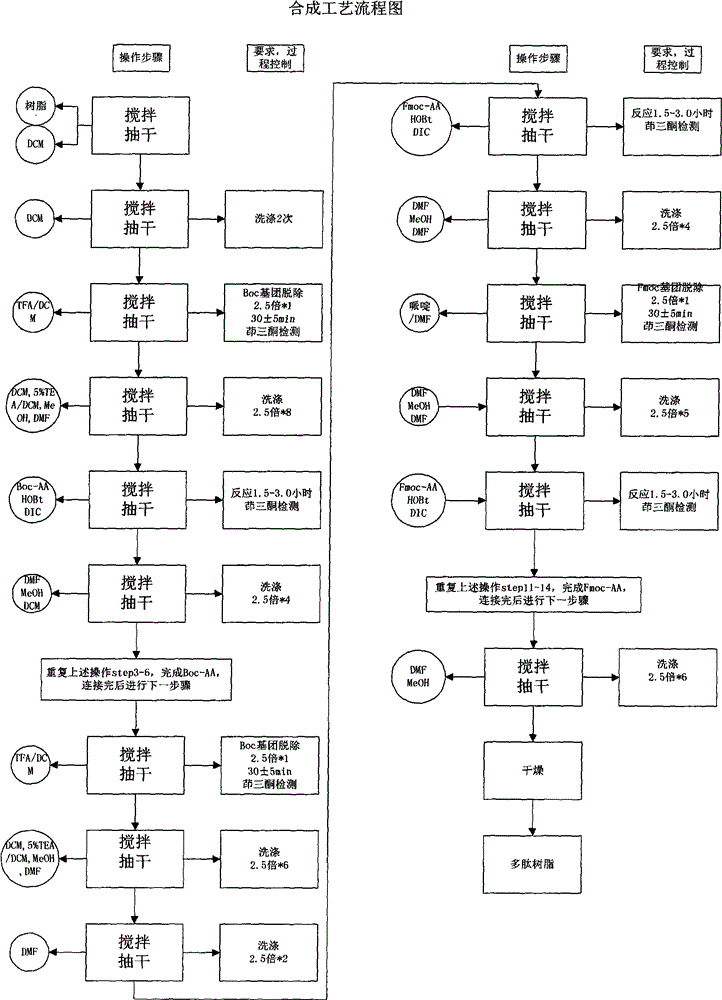
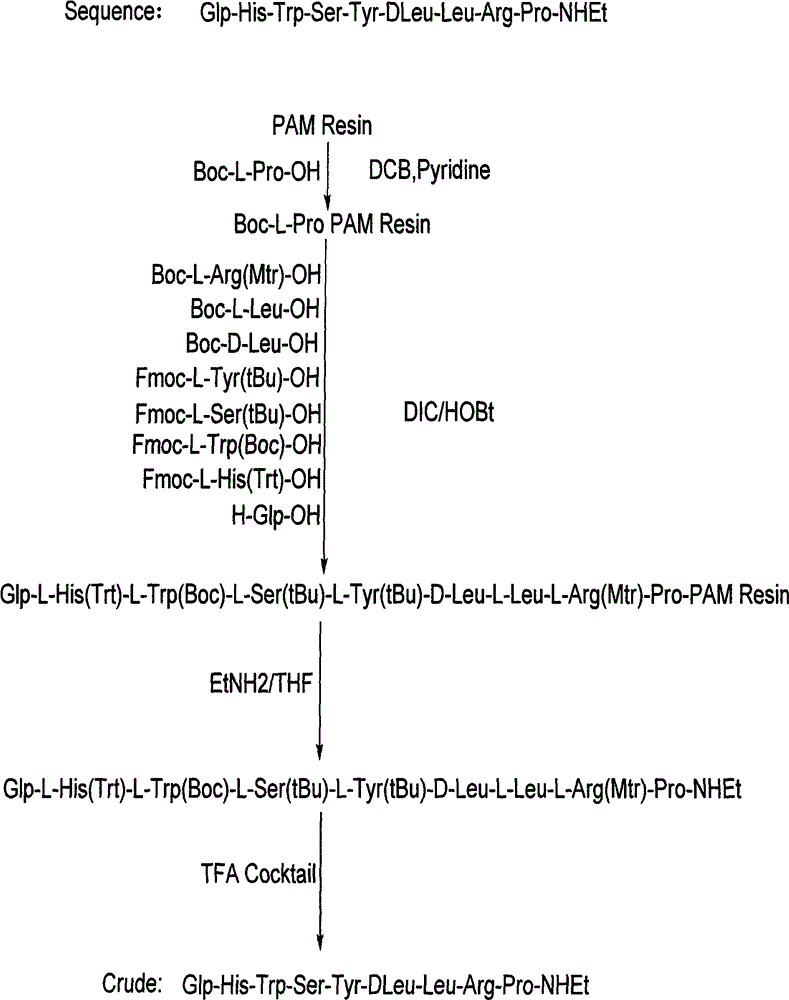
[0021] Synthesis of Resin Boc-L-Pro PAM Resin
[0022] Put PAM Resin (12.5g, degree of substitution 0.80mmol / g) in the reactor, dissolve 4.3g Boc-L-Pro-OH with 100mL of DCM, then add it into the reactor, keep stirring, and then slowly add 6.5mL of pyridine , then slowly add 5.8 mL of 2,6-dichlorobenzoyl chloride, stir for 3-6 hours, drain the filtrate, add 2.5 times the volume of the resin in DCM, wash twice for 3 minutes each time, add 2.5 times the volume of the resin in DMF, Wash 2 times, 3 minutes each time, add 2.5 times the resin volume of MeOH, wash 2 times, 3 minutes each time. Vacuum dry, first air dry for 20-30 minutes, then vacuum dry for more than 12 hours, then it can be used in step 2.
Embodiment 2
[0024] Polypeptides were synthesized and purified using Boc-L-Pro-PAM Resin.
[0025] Step 1. Boc Pro-PAM Resin resin (15.4g; degree of substitution 0.65mmol / g) is placed in the reactor, swelled with 150mL of DCM for not less than 2h, then washed twice with DCM of 2.5 times the volume of the resin, each about 2 minutes each time. The solution was removed by suction filtration. It can be used for step 2.
[0026]Step 2. Add 2.5 times the resin volume of 50% TFA / DCM solution, stir for 30±5min, remove the reaction solution by suction filtration, add 2.5 times the resin volume of DCM to the above resin to wash for 2min, remove the reaction solution, add 2.5 times the resin Wash with 5% TEA / DCM by volume for 2 minutes, remove the reaction solution, add 2.5 times the volume of the resin to wash with MeOH for 2 minutes, remove the reaction solution, add 2.5 times the volume of the resin to wash with 5% TEA / DCM for 2 minutes, remove the reaction solution, add Wash with 2.5 times th...
Embodiment 3
[0056] Polypeptides were synthesized and purified using Boc-L-Pro-PAM Resin.
[0057] Step 1. Boc-Pro-PAM Resin resin (2155.3 g, 1250 mmol) was placed in the reactor, and 20 L of DCM was added to swell for 2 hours and 6 minutes. Wash 2 times with DCM, about 2 minutes each. Remove the solution by suction filtration, which can be used in step 2
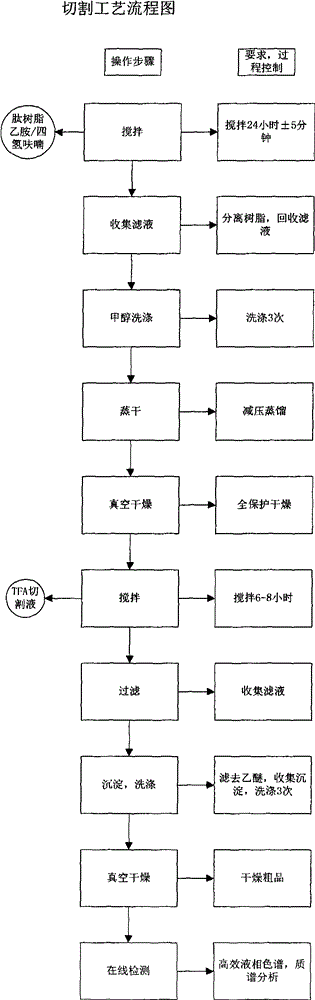
[0058] Step 2. Add 30L of 50% TFA / DCM, react for 30 minutes, remove the reaction solution by suction filtration, and then wash repeatedly with DCM, 5% TEA / DCM, methanol, and DMF in sequence, and the ninhydrin test is positive.
[0059] Weigh 1216.6g of Boc-Arg(Mtr)-OH and 337.9g of HOBt, then dissolve them in 2.3L of DMF, cool in an ice bath to less than 10°C, add 392mL of DIC to activate, activate for 23min, activate at a temperature of 14.8°C, and react for 2 hours and 40 Minutes later, the ninhydrin test was negative. The reaction solution was removed by suction filtration, and then washed repeatedly with DMF, methanol, and DCM ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com