Electrolyte with polyanion functional group and preparation method and application thereof
A polyanion and functional group technology, which is applied in the field of electrolytes with polyanion functional groups and its preparation, can solve the problems of reduced ionic conductivity of the electrolyte, influence of battery capacity and Coulombic efficiency, difficult dissociation, etc., and achieves reduction of lattice energy, Improvement of electrochemical and thermal stability, effect of effective interface
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1) Electrolyte preparation
[0039] The electrolyte solution was prepared in a glove box, which was filled with high-purity argon, the moisture was controlled below 5 ppm, and the temperature was room temperature. Ethylene carbonate (EC), dimethyl carbonate (DEC), and ethyl methyl carbonate (EMC) were purified and mixed in a mass ratio of 30:30:40, and impurities and water were removed to obtain a mixed solvent system, and then 1mol / L lithium hexafluorophosphate (LiPF 6 ) was dissolved in a mixed solvent, and an electrolyte was obtained by adding 1% VC and 1% compound 1 at the same time, and the conductivity of the electrolyte was tested.
[0040] 2) Preparation of positive pole piece
[0041] Mix the positive electrode active material LiNi according to the mass ratio of 95:3:2 1 / 3 co 1 / 3 mn 1 / 3 o 2 , the conductive agent Super-P and the binder PVDF (HSV900), and then uniformly disperse them in the solvent NMP to obtain the positive electrode slurry. The slurry ...
Embodiment 2
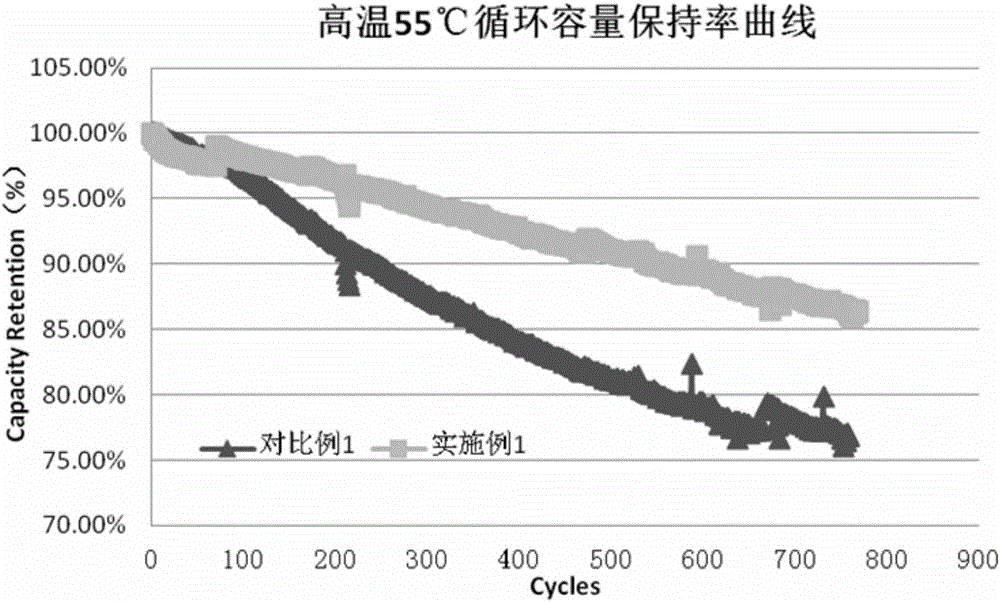
[0055] Except that 1% of compound 1 was replaced by 1% of compound 2 in the preparation of the electrolyte, the same as in Example 1, the data of normal temperature cycle, high temperature cycle and high temperature storage were tested.
Embodiment 3
[0057] Except that 1% of compound 1 was replaced by 1% of compound 3 in the preparation of the electrolyte, the same as in Example 1, the data of normal temperature cycle, high temperature cycle and high temperature storage were tested.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com