Synthesis method of PLGA (poly(lactic-co-glycolic acid)) chemically modified material and method for preparing nanoparticles from material
A glycolic acid copolymer chemistry and glycolic acid copolymer technology, applied in the field of synthesis of polylactic acid-glycolic acid copolymer chemically modified materials, to achieve uniform particle size distribution, stable properties, smooth and complete surface
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of polylactic acid-glycolic acid copolymer chemically modified material modified by embodiment 1 gallic acid derivative
[0025] Dissolve 200mg of poly(lactic-co-glycolic acid) (PLGA) completely in 10mL of dichloromethane, stir in ice bath for 20min until uniform, then add 2.3mg of N-hydroxysuccinimide, 500μL of triethyl Amine and 3.8 mg of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride were gradually heated to room temperature for 10 hours. Take the gallic acid derivative (C 26 h 21 f 6 N 3 o 4 , whose structure is as figure 1 shown), which was dissolved in 1 mL of dichloromethane to obtain a dichloromethane solution of gallic acid derivatives. Add the dichloromethane solution of gallic acid derivatives dropwise to the above reaction system, and continue to react for 20 hours at room temperature. Washing with an aqueous solution, drying overnight with anhydrous sodium sulfate, and then filtering, removing the solvent from the filtrate und...
Embodiment 2
[0029] The preparation of embodiment 2 nanoparticles
[0030] Dissolve 60 mg of the gallic acid derivative-modified polylactic-co-glycolic acid copolymer chemical modification material prepared in Example 1 in 3 mL of dichloromethane, and add it to 15 mL of a 1% polyvinyl alcohol aqueous solution, Mix evenly to obtain a mixed solution, process the mixed solution with a probe-type ultrasonic instrument for 5 minutes to form a nanoemulsion (ultrasonic power is 800W), then magnetically stir for 4 hours, remove the organic solvent in the nanoemulsion, make it completely solidified to form nanoparticles, After 10min (10000r / min) high-speed centrifugation, washing and drying, the nanoparticle with the polylactic acid-glycolic acid copolymer modified by the gallic acid derivative as the carrier is obtained.
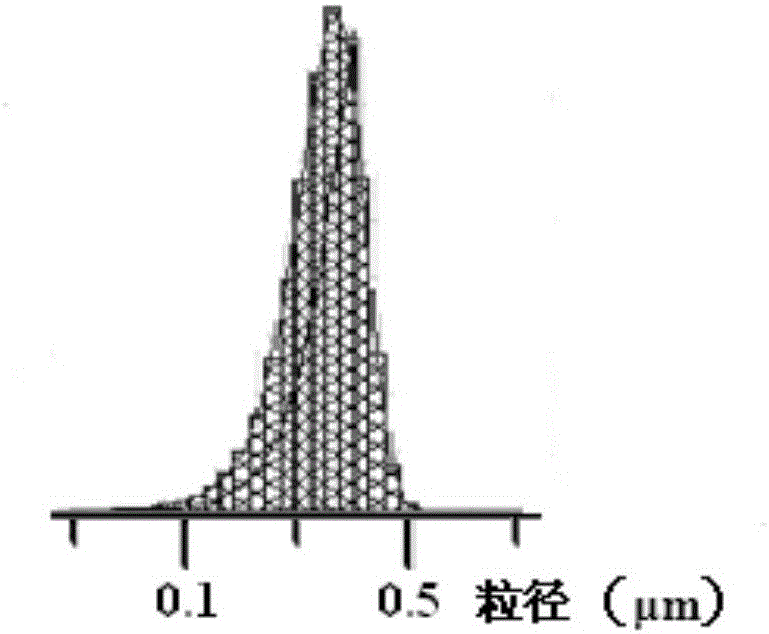
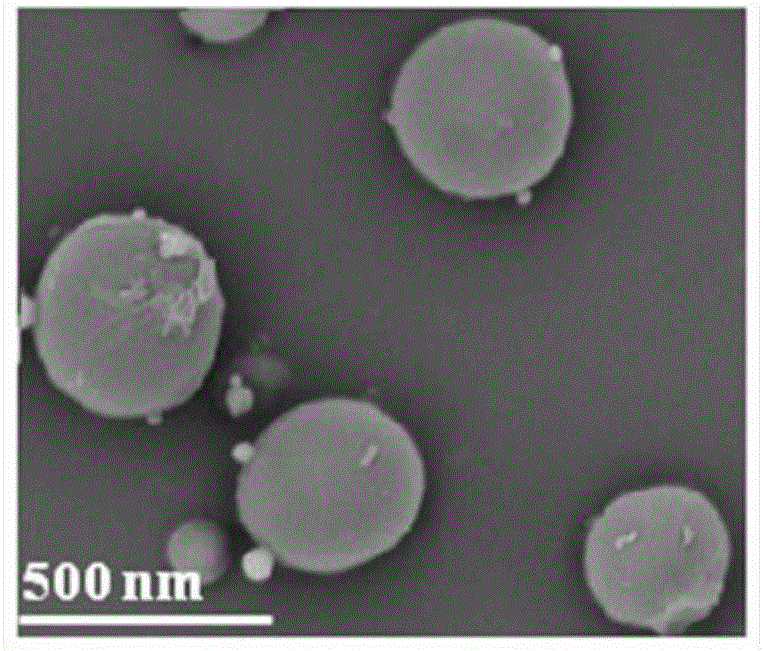
[0031] The morphology of the nanoparticles was observed under a scanning electron microscope, such as image 3 As shown, it can be seen that the surface of the nanoparticles is...
Embodiment 3
[0032] Synthesis of polylactic acid-glycolic acid copolymer chemically modified material modified by embodiment 3 gallic acid derivatives
[0033] Dissolve 200mg of poly(lactic-co-glycolic acid) (PLGA) completely in 10mL of dichloromethane, stir in ice bath for 20min until uniform, then add 2.3mg of N-hydroxysuccinimide, 500μL of triethyl Amine and 3.8 mg of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride were gradually heated to room temperature for 11 hours. Take the gallic acid derivative (C 26 h 21 f 6 N 3 o 4 , whose structure is as figure 1 shown), which was dissolved in 1 mL of dichloromethane to obtain a dichloromethane solution of gallic acid derivatives. Add the dichloromethane solution of gallic acid derivatives dropwise to the above reaction system, and continue to react for 24 hours at room temperature. Washing with an aqueous solution, drying overnight with anhydrous sodium sulfate, and then filtering, removing the solvent from the filtrate un...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com