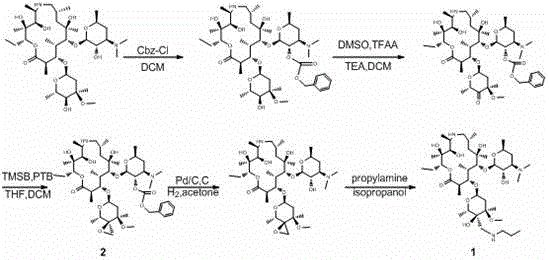
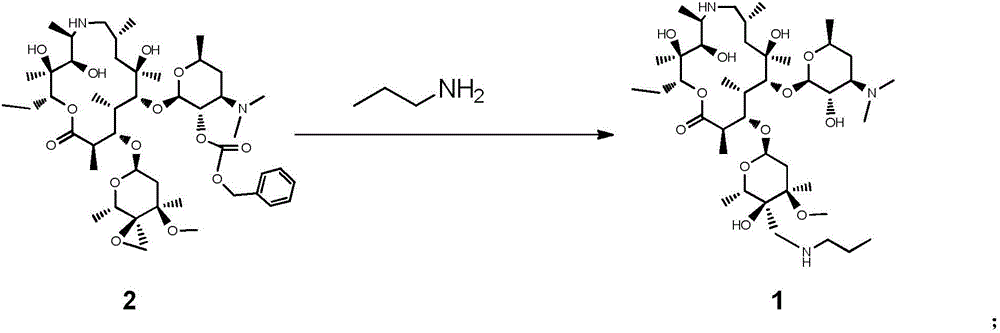
Tulathromycin A synthesis method
The technology of a kind of telamycin, synthesis method, is applied in the synthetic field of telamycin A, can solve the problems such as long reaction time, many unsafe factors, large amount of catalyst palladium carbon, etc., achieve simple and safer operation, avoid adding Hydrogen reaction, effect of improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]Put 20 g of epoxide (compound 2), 80 g of isopropanol and 20 g of n-propylamine into a 250 mL three-necked flask, start stirring, and react at 20 °C after the dissolution is complete, and react at 20 °C for 48 h. Add 60 g of methylamino alcohol solution into the reaction flask, and react at 40°C for 24 h, and the reaction is monitored by HPLC. After the reaction finishes, all solvents and excess deprotection reagents are distilled off under reduced pressure, and 100 g of methyl tert-butyl ether is added to the residue, stirred and dissolved completely, and 6.1 g of trifluoroacetic acid and 60 g of methyl tert-butyl ether are added dropwise. The mixture was stirred for 4 h, filtered, and dried to obtain the trifluoroacetic acid salt of telamycin. Add 140 mL of dichloromethane to the trifluoroacetic acid salt, add potassium carbonate aqueous solution (potassium carbonate 8.93 g, water 140 mL) under stirring, and separate layers after stirring for 30 min, add 140 mL of dich...
Embodiment 2
[0037] Put 20 g of epoxide (compound 2), 80 g of n-butanol and 30 g of n-propylamine into a 250 mL three-necked flask, start stirring, and heat up the oil bath to the internal temperature of 40 °C after the dissolution is complete, and react at 40 °C for 24 h. Add 60 g of methylamine aqueous solution into the reaction bottle, and react at 40 °C for 24 h, and the reaction is monitored by HPLC. After the reaction was completed, all solvents and excess deprotection reagents were distilled off under reduced pressure, and 100 g of ethanol was added to the residue, stirred and dissolved completely, and a mixture of 6.1 g of phosphoric acid and 60 g of methyl tert-butyl ether was added dropwise, and stirred for 4 h. Filter and dry to obtain the phosphate of telamycin. Add 140 mL of dichloromethane to the phosphate, add aqueous potassium carbonate solution (8.93 g of potassium carbonate, 140 mL of water) while stirring, and separate layers after stirring for 30 min, add 140 mL of dic...
Embodiment 3
[0039] Put 20 g of epoxide (compound 2), 100 g of methanol and 30 g of n-propylamine into a 250 mL three-necked flask, start stirring, and after the dissolution is complete, heat the oil bath to an internal temperature of 30 °C, and react at 30 °C for 24 h. Add 40 g of methylethylamine solution into the reaction bottle, and react at 30 °C for 12 h, and the reaction is monitored by HPLC. After the reaction finishes, all solvents and excess deprotection reagents are distilled off under reduced pressure, and 100 g of methyl tert-butyl ether is added to the residue, stirred and dissolved completely, and 6.1 g of trifluoroacetic acid and 60 g of methyl tert-butyl ether are added dropwise. The mixture was stirred for 4 h, filtered, and dried to obtain the trifluoroacetic acid salt of telamycin. Add 140 mL of dichloromethane to the trifluoroacetic acid salt, add potassium carbonate aqueous solution (potassium carbonate 8.93 g, water 140 mL) under stirring, and separate layers after s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com