Stabilizer for tetrachloroethylene and preparation method of stabilizer
A technology of tetrachlorethylene and stabilizer, applied in the disproportionation separation/purification of halogenated hydrocarbons, organic chemistry, etc., can solve the problems of poor moisture resistance, high corrosion rate of equipment, poor thermal stability, etc., and achieve good moisture resistance and corrosion resistance Low efficiency and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 A kind of stabilizer for tetrachlorethylene
[0040] Comprising the following components by weight:
[0041] Triethanolamine 58g, n-pentane 50g, butylchlorohydrin 50g, butylene oxide 50g, thymol 5g,
[0042] The consumption of described stabilizer in tetrachlorethylene is 0.0213%;
[0043] The triethanolamine is produced by the Laiyang Economic and Technological Development Zone Fine Chemical Factory.
[0044] The n-pentane is produced by Shanghai Lingfeng Chemical Reagent Co., Ltd.
[0045] The thymol is produced by Tianjin Institute of Chemical Reagents.
[0046] The butylene oxide is an imported product from Japan.
[0047] Described butylchlorohydrin is imported product with original packaging from Japan.
[0048] The quality requirement of each composition in stabilizer of the present invention:
[0049] Table 1 Triethanolamine quality index
[0050]
[0051] Table 2 n-pentane quality index
[0052]
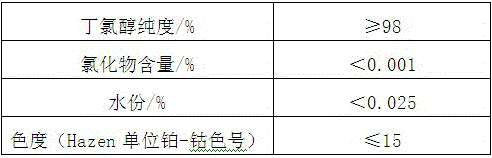
[0053] Table 3 Quality index of butad...
Embodiment 2
[0076] Embodiment 2 A kind of stabilizer for tetrachlorethylene
[0077] Comprising the following components by weight: 64 g of triethanolamine, 80 g of n-pentane, 80 g of butylchlorohydrin, 80 g of butylene oxide, and 5 g of thymol;
[0078] The consumption of described stabilizer in perchlorethylene is 0.0309%;
[0079] The triethanolamine is produced by the Laiyang Economic and Technological Development Zone Fine Chemical Factory.
[0080] The n-pentane is produced by Shanghai Lingfeng Chemical Reagent Co., Ltd.
[0081] The thymol is produced by Tianjin Institute of Chemical Reagents.
[0082] The butylene oxide is an imported product from Japan.
[0083] Described butylchlorohydrin is imported product with original packaging from Japan.
[0084] The requirement of stabilizer for tetrachlorethylene of the present invention to tetrachlorethylene:
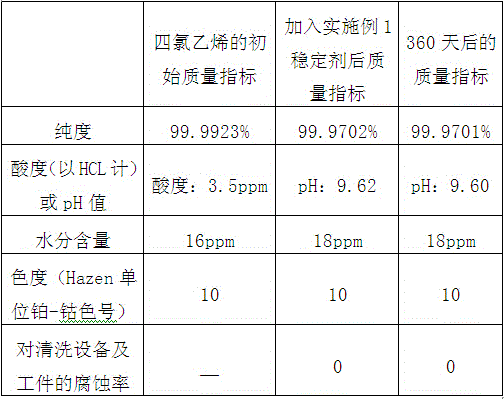
[0085] Raw material tetrachlorethylene purity ≥ 99.99%;
[0086] Raw material tetrachloroethylene acid value (calculated ...
Embodiment 3
[0097] Embodiment 3 a kind of stabilizer for tetrachlorethylene
[0098] Comprising the following components by weight: 70 g of triethanolamine, 100 g of n-pentane, 100 g of butychlorohydrin, 100 g of butylene oxide, and 5 g of thymol;
[0099] The consumption of described stabilizer in perchlorethylene is 0.0375%;
[0100] The triethanolamine is produced by the Laiyang Economic and Technological Development Zone Fine Chemical Factory.
[0101] The n-pentane is produced by Shanghai Lingfeng Chemical Reagent Co., Ltd.
[0102] The thymol is produced by Tianjin Institute of Chemical Reagents.
[0103] The butylene oxide is an imported product from Japan.
[0104] Described butylchlorohydrin is imported product with original packaging from Japan.
[0105] The requirement of stabilizer for tetrachlorethylene of the present invention to tetrachlorethylene:
[0106] Raw material tetrachlorethylene purity ≥ 99.99%;
[0107] Raw material tetrachloroethylene acid value (calculate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com