Source tracing method for bacteria in infant formula milk powder
An infant formula and bacteria technology, applied in the field of food safety, can solve the problems of single detection and single prevention and control of bacteria, traceability of finished product bacteria source, pollution and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 sample collection
[0030] All the samples used in the research (raw material samples, air samples, surface coating samples) were collected from an infant formula milk powder processing plant in winter (November 2014) and summer (June 2015) by our laboratory staff respectively. factory. See Table 3 for the sample information of raw and auxiliary materials, and three samples were collected for each type of sample.
[0031] Table 3 Raw and auxiliary materials of infant formula milk powder
[0032]
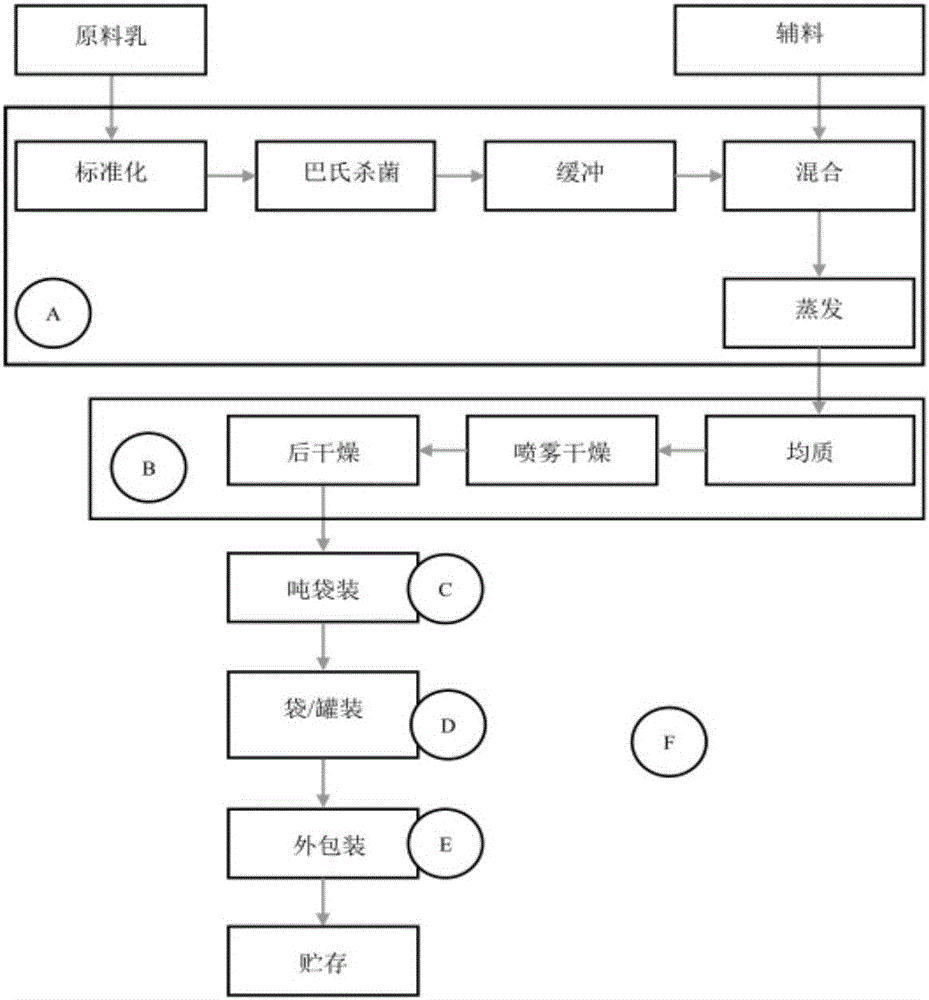
[0033] For air sample and surface smear sample collection areas, see infant formula milk powder processing flow chart figure 1 , a total of 6 sampling areas, including 5 indoor areas and 1 outdoor area. Among them, the indoor area covers the main links of the infant formula milk powder processing process, including the pre-treatment link (A), the production link (B), the production in-package link (C), the packaging in-package link (D), and the packaging link (...
Embodiment 2
[0036] The separation of embodiment 2 bacteria:
[0037] Samples of raw materials
[0038] Weigh 25g of raw and auxiliary material samples of infant formula milk powder into a sterilized 225mL BPW Erlenmeyer flask, mix thoroughly, take 1mL of the mixed solution and add it to 9mL sterile PBS, after mixing, take 1mL and add it to another 9mL sterile In PBS, carry out gradient dilution by analogy, select the appropriate dilution gradient, absorb 100 μL of the mixed solution for each dilution gradient, and evenly spread it on the TSA solid medium, each sample is 3 parallel, and cultivate it in a constant temperature incubator at 37°C for 24 hours , select a single colony with different appearances in LB liquid medium, mix them and culture them in a 37°C incubator for 24h. Purification is required for non-single colonies. First, use the inoculation loop to dip the shaken overnight cultured LB bacterial solution, line the TSA solid medium with three zones, and incubate it upside do...
Embodiment 3
[0042] Embodiment 3 bacterial identification:
[0043] Genomic DNA Extraction
[0044] For the raw material samples of infant formula milk powder and surface smear samples, 2 mL of LB liquid bacterial liquid of the isolated strains were taken respectively, and the DNA of the isolated strains was extracted according to the specific method required by the TIANamp Bacterial Genomic DNA Extraction Kit.
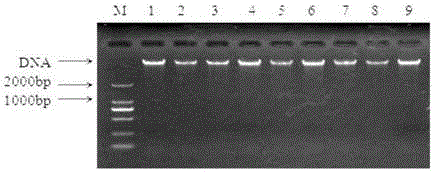
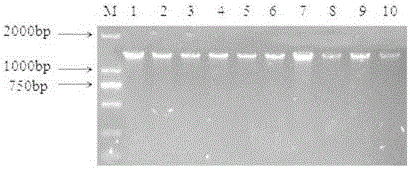
[0045] Utilize bacterial genomic DNA extraction kit to carry out DNA extraction to isolated bacteria, and detect on 1% agarose gel electrophoresis, some results are as follows figure 2 shown.
[0046] It can be seen from the figure that the extracted DNA of the isolated strain has only one clear band without interference from other bands, indicating that the DNA extraction results meet the requirements and can be used for subsequent amplification experiments.
[0047] PCR amplification system and cycle conditions
[0048](1) PCR amplification of 16S rDNA sequence
[0049] Amp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com