Pancreas cancer prevention use of polycyclic fused macrocyclic lactam compounds
A macrocyclic lactam and compound technology, applied in the field of natural medicinal chemistry, can solve the problems of non-negligible toxic effects and restricting drug efficacy, and achieve good market application and promotion prospects, high lethality, and clear activity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1, Structural Identification of Polycyclic Fused Macrocyclic Lactam Compounds
[0027] Get S.xiamenensis M6 bacterial strain (being Streptomyces xiamenensis (Streptomyces xiamenensis) CGMCC No.4.3534 and carry out liquid fermentation culture (30 liters), 7 days, supernatant after centrifugation is extracted 3 times with ethyl acetate. The extracts are combined, concentrated , to get 3 grams of total extract.Get the total extract and add 9 grams of reverse phase mixing samples after dissolving with an appropriate amount of chloroform-methanol mixed solvent, go up to the normal phase silica gel glass decompression column that is packed with 50 grams, wash with methanol-water gradient The removal is specifically: 50%, 80%, 100% methanol-water gradient elution, each gradient elution volume is 200mL, 150-100ml / bottle is received, and the polarity of the elution solvent is adjusted by increasing the amount of methanol in chloroform Gradient increases, and each eluti...
Embodiment 2
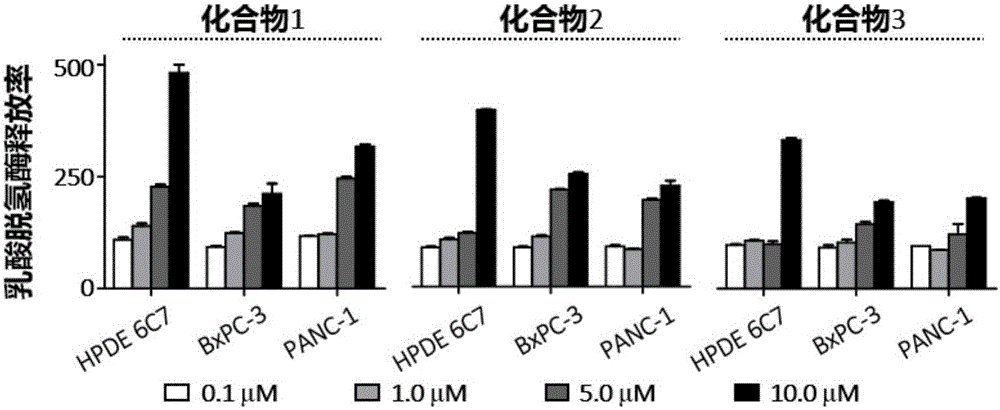
[0035] Example 2. Polycyclic fused macrocyclic lactam compounds inhibit the proliferation and viability of human pancreatic cancer cells
[0036] The materials are as follows:
[0037] Cells: human pancreatic cancer cells PANC-1, BxCP1; drug: compound 1 obtained from the above examples. Among them, compound 1 was used as the drug-treated experimental group, and compound 2 was used as the drug-treated comparison group.
[0038] Method: PANC-1, BxCP1 cells were seeded in 96-well plate, 0.5×10 per well 3 cells. After 24 hours, the liquid was changed and drugs were added, the control group was DMSO, and the final concentration was 1 / 1000. Cell viability was measured 0, 1, 2, 3, 4, 5, and 6 days after adding the drug. The measurement method is aspirating out the medium, adding 100 μl of complete medium and 10 μl of CCK-8 reagent to each well, and incubating in the incubator for 60 minutes to measure the light absorption value with a microplate reader at a wavelength of 450 nm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com