Preparation method and anti-pulmonary fibrosis application of benzopyran compound
A technology for benzopyran compounds and pulmonary fibrosis, which is applied in the field of natural medicinal chemistry, can solve problems such as no benzopyran compounds reported in literature, achieve good market application and promotion prospects, and have low toxicity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
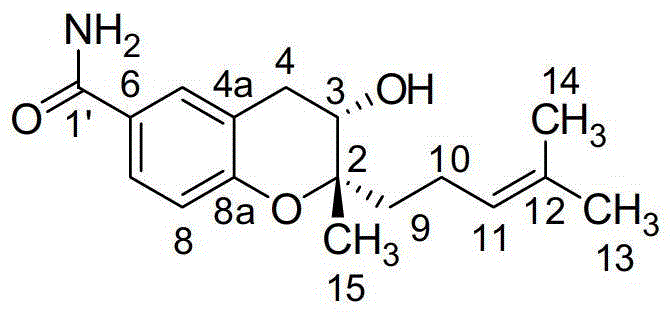
[0033] Embodiment 1, separation and purification of benzopyran compound
[0034]Take the S.xiamenensis M6 strain (i.e. Streptomyces xiamenensis CGMCC NO.5675) for liquid fermentation culture (30 liters) for 7 days. After centrifugation, the supernatant was extracted three times with ethyl acetate, and the residue was extracted with an equal volume of Ethyl acetate: methanol: formic acid = 80:15:5 (volume ratio) mixed solvent extraction 3 times, 12 hours each time. The extracts were combined and concentrated to obtain 13.4 g of the total extract. Get the total extract and dissolve it with an appropriate amount of chloroform-methanol mixed solvent, add 10 grams of 200-300 mesh silica gel G (product of Qingdao Ocean Chemical Group Co., Ltd.) - methanol (100:1, 70:1, 60:1, 50:1, 30:1, 15:1, 10:1, 5:1, 2:1, 1:1, (volume ratio) to methanol] Gradient elution, the flow rate is 15 seconds / drop, 150-100 ml / bottle receiving, the polarity of the elution solvent is gradually increased by...
Embodiment 2
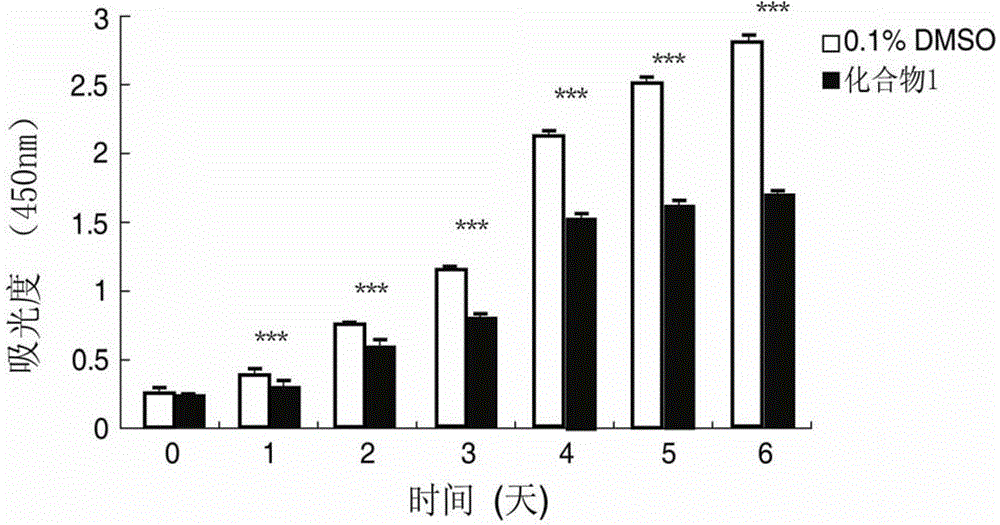
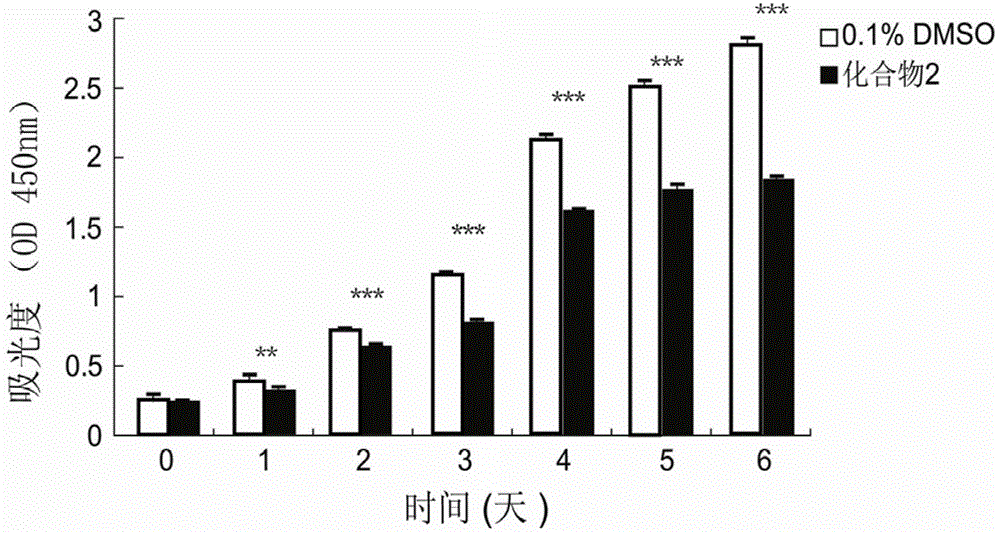
[0039] Example 2. Benzopyran Compounds Inhibit the Proliferation and Viability of Human Lung Fibroblasts
[0040] The materials are as follows:
[0041] Cells: Human lung fibroblasts (human lung fibroblasts, W126 cells); Drugs: Compound 1 (Xiamenmycin C) and Compound 2 (Xiamenmycin) obtained from the above examples Among them, compound 1 was used as the drug-treated experimental group, and compound 2 was used as the drug-treated comparison group.
[0042] Method: WI26 cells grown to 70-80% confluent were digested with 0.25% trypsin and seeded in 96-well plate, 1×10 per well. 3 cells. After 24 hours, the medium was changed and drugs were added. The final concentration of compound 1 in the drug treatment experimental group was 15 μg / ml, and the solvent DMSO concentration was 1 / 1000. The control group was DMSO, and the final concentration was 1 / 1000. The final concentration of compound 2 in the drug treatment control group was 30 μg / ml, and the solvent DMSO concentration was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com