Tumor cell targeting mesoporous silicon nanometer assembly and preparation method for same
A nano-assembly and tumor cell technology, applied in the field of mesoporous silicon nano-assembly and its preparation, can solve the problems of drug leakage, side effects, uncontrollable drug release, etc., to promote uptake, enhance drug concentration, and solve controllable release poor sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation method of lipid mesoporous silicon core-shell nano-assembly modified by hyaluronic acid
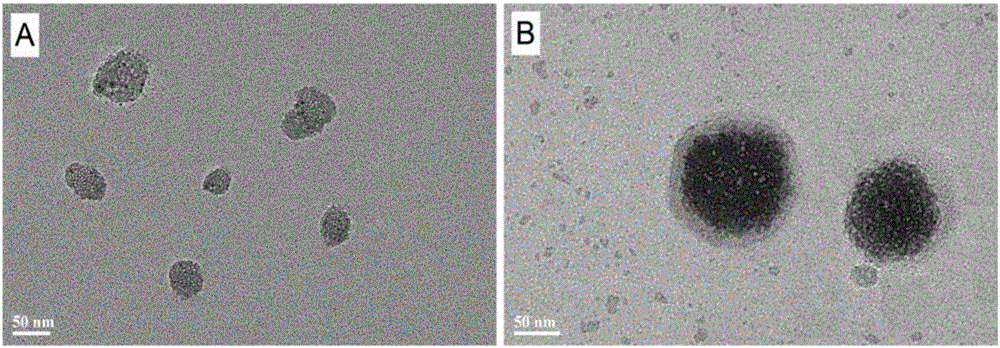
[0026] (1) Preparation of mesoporous silicon nanoparticles: Take 3.6mL of 2mol / L NaOH solution, add it to 800mL water, heat to 80°C, and then dissolve 2.4g of cetyltrimethylammonium bromide CTAB in ethanol The solution was added into hot water at 80° C., and reacted for 2 hours at a rotation speed of 700 rpm. Then slowly add tetraethyl orthosilicate TEOS 8mL, and continue to react for 8h. Add an equal volume of ethanol to the reaction system, and after a flocculent precipitate precipitates, filter, dissolve the precipitate in a mixture of ethanol: hydrochloric acid = 9:1, and reflux at 80°C for 8 hours to effectively remove the surfactant , and then centrifuged and dried to obtain mesoporous silicon nanoparticles (MSNs) with an average particle diameter of about 85 nm.
[0027] (2) Disperse the prepared mesoporous silicon with ethanol, mix it with 10 mg / mL doxorubicin...
Embodiment 2
[0035] Preparation method of lipid mesoporous silicon core-shell nano-assembly modified by hyaluronic acid
[0036] (1) Prepare mesoporous silicon nanoparticles according to the method described in Example 1, disperse the prepared mesoporous silicon with ethanol, mix it with 15mg / mL doxorubicin (DOX) solution, and keep away from light Stir for 8h to make full contact. Centrifuge after 8 hours, wash with PBS buffer several times, and when the washing solution is colorless, the drug-loaded mesoporous silicon DOX@MSNs are obtained. The washing buffer and the residual DOX solution were collected, and the DOX concentration of the residual solution was measured using an ultraviolet spectrophotometer at a wavelength of 480 nm, and then the drug loading capacity of MSNs was obtained. It was determined that the final drug loading capacity of MSNs was 12.68mg, that is, 12.68mg DOX was loaded per 100mg MSNs.
[0037](2) Weigh 30 mg of hydrogenated soybean lecithin, 7.5 mg of cholestero...
Embodiment 3
[0042] (1) According to Example 1, the prepared mesoporous silicon was dispersed with ethanol, mixed with 10-hydroxycamptothecin solution, and stirred for 24 hours in the dark to make it fully contact. After 8 hours, it was centrifuged, washed with PBS buffer, and dried in vacuum to obtain MSNs loaded with 10-hydroxycamptothecin, which were stored in the dark at room temperature.
[0043] (2) Weigh 25 mg of hydrogenated soybean lecithin, 6.25 mg of cholesterol and 1.64 mg of distearoylphosphatidylethanolamine, respectively, and dissolve them in 20 mL of chloroform. Rotary steam at 30°C to form a film. The lipid film was hydrated with 40 mL of PBS buffer dissolved in 10 mg of mesoporous silicon, and hydrated using a rotary evaporator under a water bath condition of 60 °C. The homogenizer was homogenized under high pressure for 5 minutes under the condition of 1000 Pa to obtain liposomes loaded with mesoporous silicon.
[0044] (3) Weigh 26mg HA (600-800KDa) into 10mL distille...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com