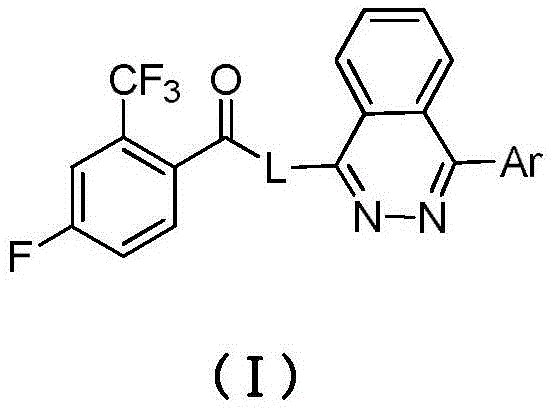
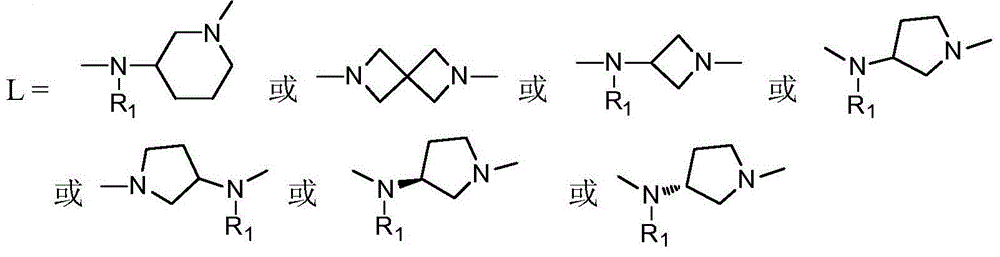
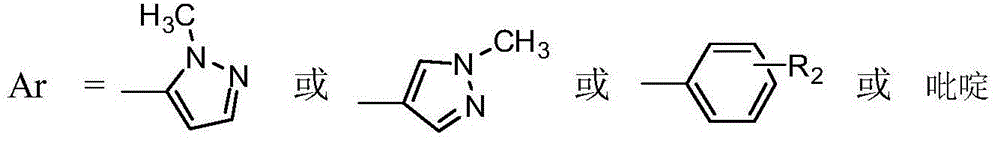
Arylphthalazine compound and its preparation method and use
A compound, aryl technology, applied in the field of drug synthesis, can solve the problems of anti-tumor drug resistance, reduce CML cells, reduce the growth of imatinib-resistant CML cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of compound 1, N-(1-(4-(1-methyl-pyrazol-5-yl)-phthalazin-1-yl)piperidin-3-yl)-4-fluoro-2 -Trifluoromethylbenzamide
[0029] 1) Synthesis of N-(1-benzyl-piperidin-3-yl)-4-fluoro-2-trifluoromethylbenzamide
[0030] In a 50mL eggplant-shaped bottle, add 2-trifluoromethyl-4-fluorobenzoic acid (546mg, 2.63mmol), ultra-dry THF (10mL), EDC . HCl (1.0g, 5.26mmol), stirred at room temperature for 0.5h, added DMAP (65mg, 0.53mmol), replaced the air in the reaction system with argon, and added dropwise the compound N-benzyl-3-aminopiperidine (500mg, 2.63 mmol) in ultra-dry THF (5 mL). After the dropwise addition was completed, stir at room temperature for 16 h under argon atmosphere, TLC (PE:EA=1:1) detected that the reaction was complete, added saturated sodium bicarbonate solution (150 mL), extracted with DCM (50 mL×3), combined the organic layers, and saturated salt Wash with water (50mL×1), dry over anhydrous sodium sulfate, evaporate the solvent und...
Embodiment 2
[0037] Example 2: Preparation of compound 2, N-methyl-N-(1-(4-(1-methyl-pyrazol-5-yl)-phthalazin-1-yl)piperidin-3-yl)- 4-Fluoro-2-trifluoromethylbenzamide
[0038] 1) Synthesis of N-methyl-N-(1-tert-butoxycarbonyl-piperidin-3-yl)-4-fluoro-2-trifluoromethylbenzamide
[0039] In a 50mL eggplant-shaped bottle, add 2-trifluoromethyl-4-fluorobenzoic acid (1.5g, 7.1mmol), ultra-dry THF (25mL), EDC . HCl (1.8g, 9.3mmol), stirred at room temperature for 0.5h, added DMAP (120mg, 0.93mmol), replaced the air in the reaction system with argon, and added dropwise the compound N-tert-butoxycarbonyl-3-aminomethylpiperidine ( 1.0 g, 4.7 mmol) in ultra-dry THF (5 mL). After the dropwise addition was completed, stir at room temperature for 16 h under argon atmosphere, TLC (PE:EA=1:1) detected that the reaction was complete, added saturated sodium bicarbonate solution (50 mL), extracted with DCM (50 mL×3), combined the organic layers, and saturated salt Washed with water (50mL×1), dried over ...
Embodiment 3
[0046] Example 3: Preparation of compound 3, 2-(4-(1-methylpyrazol-5-yl)phthalazin-1-yl)-6-(2-trifluoromethyl-4-fluoro-benzoyl )-2,6-diazaspiro[3,3]heptane
[0047] 1) Synthesis of 2-tert-butoxycarbonyl-6-(2-trifluoromethyl-4-fluoro-benzoyl)-2,6-diazaspiro[3,3]heptane
[0048] In a 50mL eggplant-shaped bottle, add 2-trifluoromethyl-4-fluorobenzoic acid (1.5g, 7.1mmol), ultra-dry THF (25mL), EDC . HCl (1.8g, 9.3mmol), stirred at room temperature for 0.5h, added DMAP (120mg, 0.93mmol), replaced the air in the reaction system with argon, and added compound 11 (1.0g, 7.4mmol) in ultra-dry THF (5mL) dropwise solution. After the dropwise addition was completed, stir at room temperature for 16 h under argon atmosphere, TLC (PE:EA=1:3) detected that the reaction was complete, added saturated sodium bicarbonate solution (50 mL), extracted with DCM (50 mL×3), combined the organic layers, and saturated salt Wash with water (50mL×1), dry over anhydrous sodium sulfate, evaporate the sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com