Azithromycin dry-suspended granule with odor masked and preparation method thereof
A technology of azithromycin and dry suspension, applied in the field of new azithromycin preparations and dry suspension granules, can solve the problems of improved medication compliance, instability, and prominent drug odor of patients, so as to improve medication compliance, improve the applicable population, The effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Azithromycin taste-masked dry suspension granules
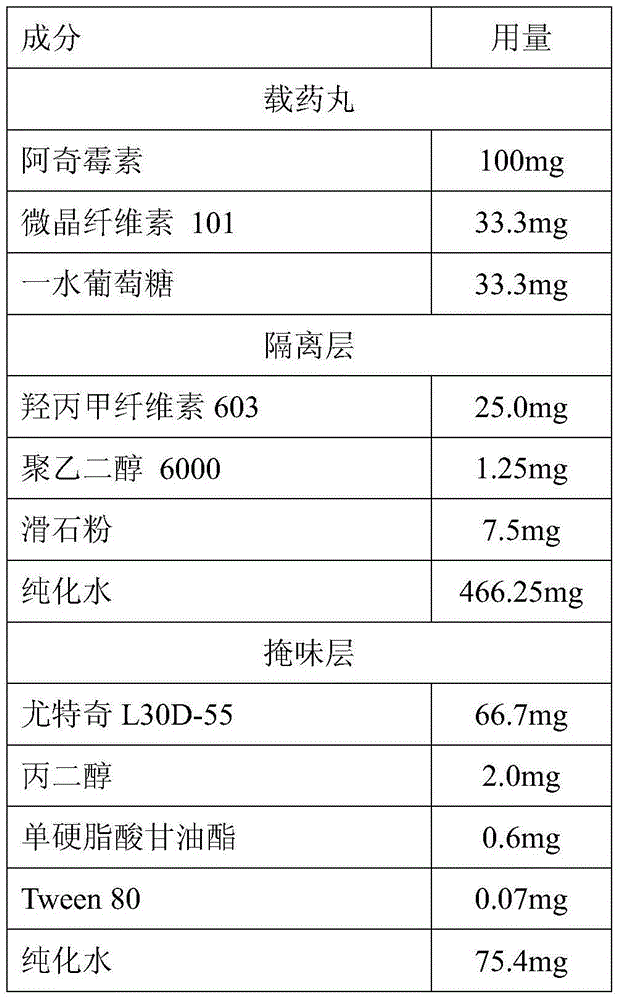
[0057] List of prescriptions per unit formulation of azithromycin taste-masked dry suspension granules:
[0058]
[0059]
[0060] making process:
[0061] 1. Preparation of loaded pills:
[0062] The drug-loaded pellets are prepared by extrusion and spheronization.
[0063] 1.1 Preparation of soft materials: Put the prescribed amount of microcrystalline cellulose 101, azithromycin, and glucose in a wet granulator, start the granulator and stir evenly, and add an appropriate amount of purified water while the materials are stirring, and the purified water Add in the form of atomization and continue to stir for a certain period of time to obtain the desired soft material.
[0064] 1.2 Extrusion of soft material: use 0.5mm extrusion screen to extrude the material to obtain an extrudate of suitable length.
[0065] 1.3 Shot blasting: When the shot blasting machine is running, add extrudates, the speed...
Embodiment 2
[0081] Embodiment 2: the azithromycin dry suspension pellet drug evaluation that embodiment 1 makes
[0082] 1. Taste evaluation method and results: According to the ratio of drug to water of 11:3, the dry suspension pellets of azithromycin prepared in Example 1 were made into dry suspension syrup. Tasted by volunteers, the taste was good without bad taste.
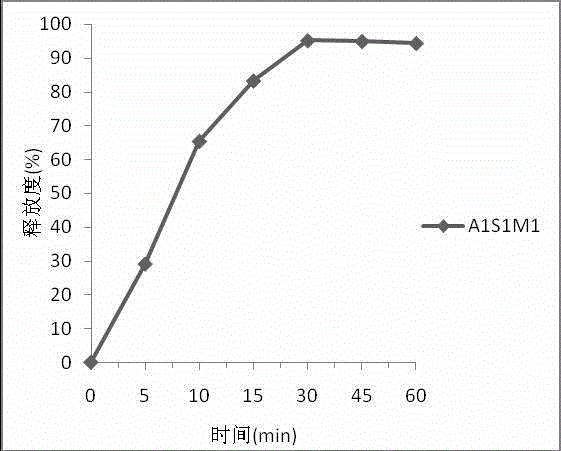
[0083] 2. Release test
[0084] (1) Determination method: take this product, according to the dissolution test method (Chinese Pharmacopoeia 2010 edition two appendix XC second method), with phosphate buffer (pH6.0) (weigh potassium dihydrogen phosphate 6.805g, hydrogen Add 0.224g of sodium oxide to 1L of degassed water, adjust to pH6.0) 900ml is the dissolution medium, the rotating speed is 75 revolutions per minute, operate according to the law, after 30 minutes, take 5ml of the solution, filter, and take the subsequent filtrate as the test sample solution. In addition, accurately weigh an appropriate amount of azithr...
Embodiment 3
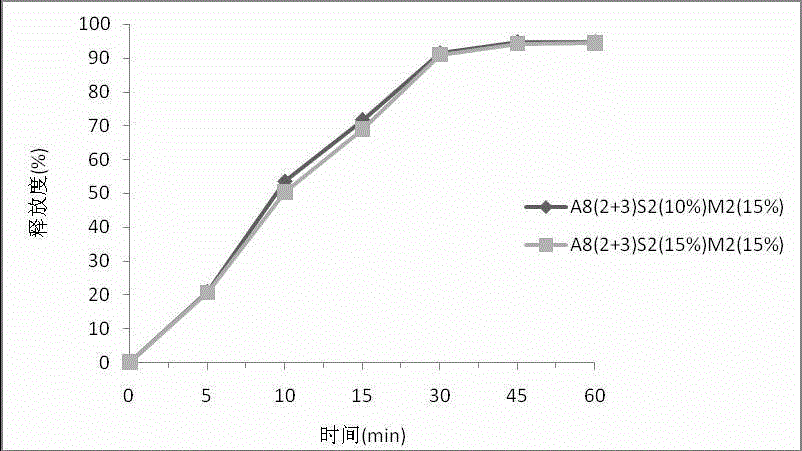
[0108] Example 3: Investigation test on the influence of the weight gain of different isolation layers on the release rate
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com