Single-domain heavy-chain antibody aiming at prostate specific membrane antigen
A single-domain heavy chain antibody, prostate-specific technology, used in tumor-specific antigens, tumor rejection antigen precursors, anti-animal/human immunoglobulins, etc. The effect of good performance, low cost and good application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Eukaryotic Expression of the Extramembrane Region of Prostate Specific Membrane Antigen
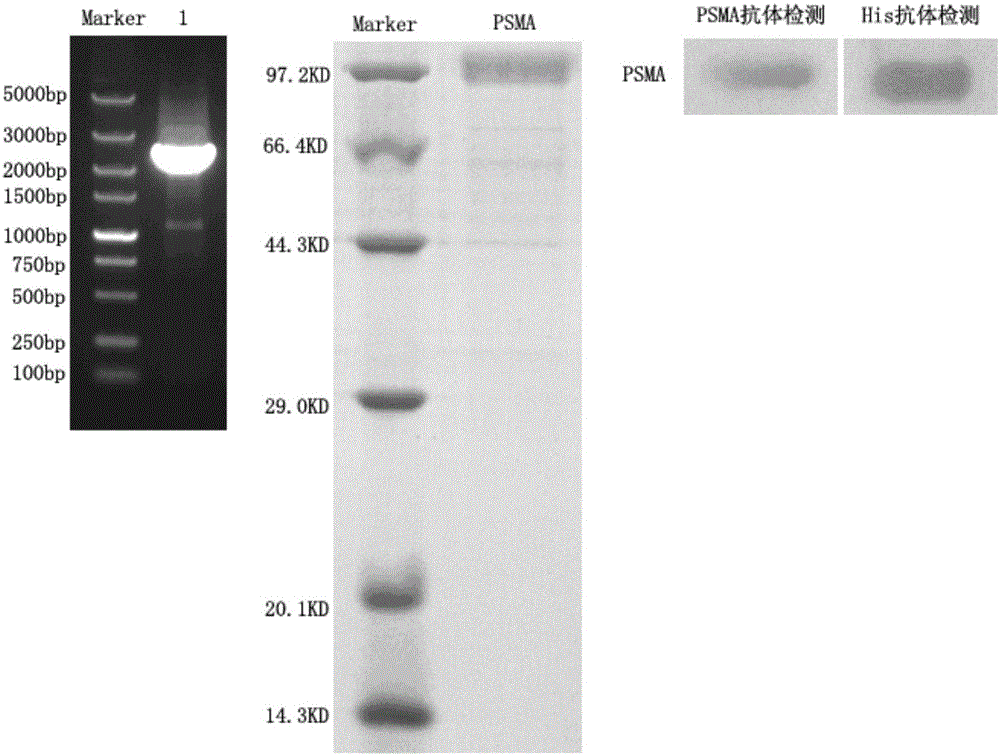
[0028] RNAiso Plus reagent was used to extract the total RNA in LNCaP cells with high expression of PSMA, and the DNA fragment encoding the extracellular region of PSMA was obtained by RT-PCR method, and it was inserted into eukaryotic expression by using Not I and BamH I restriction enzymes The vector pRAG2a was ligated into a recombinant plasmid by T4 DNA ligase. The recombinant plasmid was heat-shocked and transformed into TOP 10 competent cells and cultured overnight, and the correctly identified clones were sent for sequencing verification. The plasmids of positive clones were extracted, and the DNA plasmids were transfected into HEK-293 cells using liposome Lipofectamine 2000 for culture, and the supernatants were collected at different time points for SDS-PAGE electrophoresis detection. After culturing for a certain period of time, the protein was purified according to the ...
Embodiment 2
[0030] Panning and Identification of Anti-Prostate-Specific Membrane Antigen Single-Domain Heavy-Chain Antibody
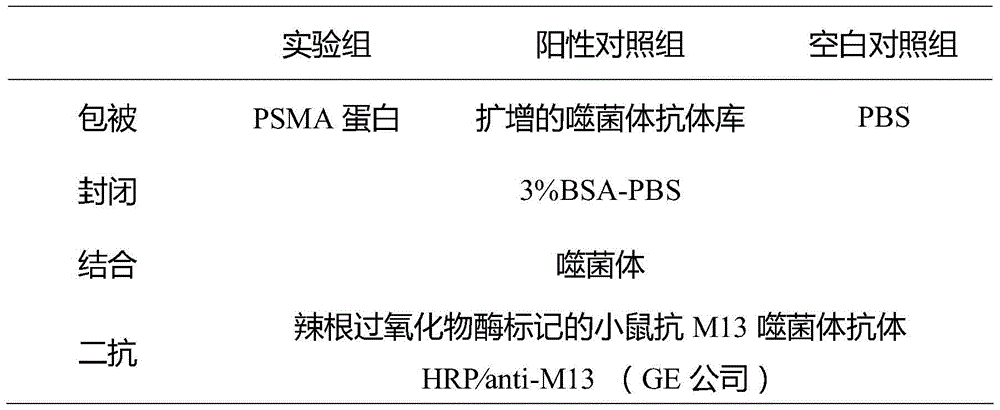
[0031] Using solid-phase panning method to display camel-derived natural antibody phage display library (for reference: "Tu Zhui, Xu Yang, Liu Xia, et al. Construction and identification of camel-derived natural single-domain heavy chain antibody library [J]. China Bioengineering Journal, 2011, 31 (4): 31-36." In the display library constructed in "), the single-domain heavy chain antibody against prostate-specific membrane antigen was panned. The expression of the extracellular region of prostate-specific membrane antigen was carried out according to the above-mentioned Example 1. During the first round of panning, the protein of the extracellular region of PSMA synthesized above was diluted to 150 μg / mL with phosphate buffered saline solution (PBS, pH7.4) (The coating concentrations of rounds 2-4 are 100, 50, and 50 μg / mL, respectively), add 100 μL to each well o...
Embodiment 3
[0040] ELISA and fluorescent immunoassay of PSMA expressing cells
[0041] Gastric cancer cell MKN45 does not express PSMA, while prostate cancer cell LNCaP expresses PSMA. Taking these two types of cells as examples, the anti-prostate specific membrane antigen single-domain heavy chain antibody phage-positive clones obtained by panning in Example 2 were used for cell-level detection. ELISA and fluorescent immunoassay. LNCaP cells (expressing PSMA) and MKN45 cells (not expressing PSMA) were seeded into 96-well plates at 1×10 4 , cultured overnight. After fixing with 4% paraformaldehyde, 100 μL of 3% hydrogen peroxide solution was added dropwise to each well to block endogenous peroxidase activity, and incubated at 37°C for 30 min. Wash the plate three times with TBS, block with 5% BSA-PBS, add 100 μL anti-prostate specific membrane antigen single domain heavy chain antibody phage-positive clone, and incubate at 37°C for 1 hour. Then rinse with PBS, add HRP-anti-M13 antibody...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com